Synthesis method of tenofovir disoproxil fumarate dimer impurity
A technology of ester fumarate dimer and tenofovir, which is applied in the field of synthesis of impurities of tenofovir disoproxil fumarate dimer, can solve the limitation of tenofovir disoproxil fumarate dimer problems such as the research process of polymer impurities, to achieve the effect of short route and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthetic method of tenofovir disoproxil fumarate dimer impurity in the present embodiment comprises the following steps:
[0031] (1) Add 5g of paraformaldehyde and 150mL of hydrochloric acid with a volume concentration of 15% to a 250mL four-neck flask at room temperature. After the addition is completed, heat up to 35-40°C and stir for 1 hour. After completely dissolving, cool down to 10-15°C. And at this temperature, add 10 g of the compound of formula II below, and keep it warm for 48 hours at the same temperature;
[0032]
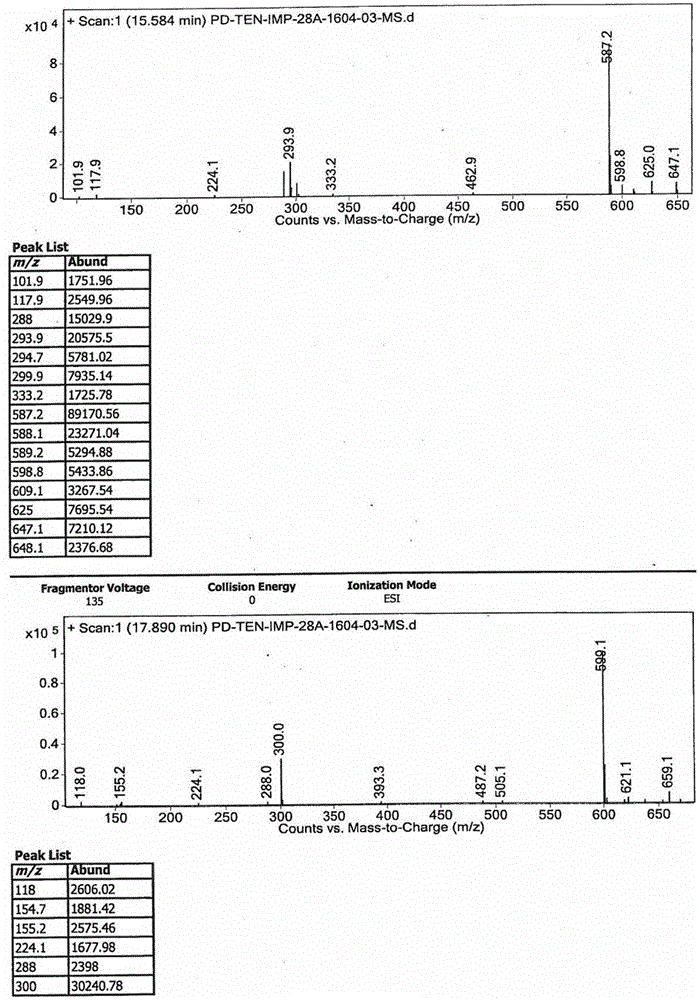
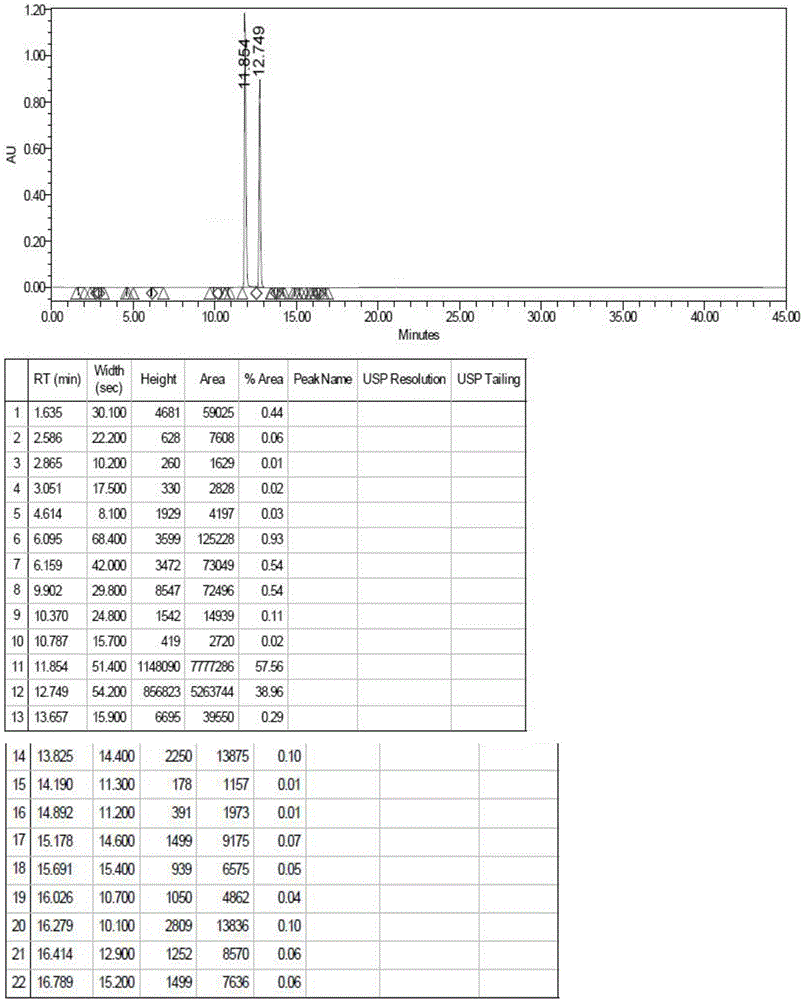
[0033] (2) step (1) completes the reaction, decompresses and evaporates hydrochloric acid to the utmost, obtains the compound of formula III; figure 1 It can be seen that the molecular weight of the product obtained in this step is consistent with the target product formula III compound; as figure 2 As shown, the purity HPLC=57.56% of the obtained formula III compound;
[0034]
[0035] (3) Add the compound of formula III obtained...
Embodiment 2
[0044] The synthetic method of tenofovir disoproxil fumarate dimer impurity in the present embodiment comprises the following steps:
[0045] (1) Add 5g of paraformaldehyde and 150mL of hydrobromic acid with a volume concentration of 15% to a 250mL four-necked flask at room temperature. 15°C and add 15g of the following compound of formula II at this temperature, and keep the reaction at the same temperature for 48h;
[0046] (2) Step (1) completes the reaction, and decompression steams and removes hydrobromic acid to the utmost, and obtains the compound of formula III;
[0047] (3) Add the compound of formula III obtained in step (2) into a 250 mL four-necked flask, and add 27 g of chloromethyl isopropyl carbonate, 30 mL of DMSO, 20 mL of triethylamine, and 5.3 g of TBAB in sequence, and stir to raise the temperature to 50-60 °C and stirred at this temperature for 4 hours, then cooled to 30-35 °C and filtered, and the obtained filtrate A was collected;
[0048] Rinse the fi...
Embodiment 3
[0050] The synthetic method of tenofovir disoproxil fumarate dimer impurity in the present embodiment comprises the following steps:
[0051] (1) Add 5g of paraformaldehyde and 150mL of hydrochloric acid with a volume concentration of 15% to a 250mL four-neck flask at room temperature. After the addition is completed, heat up to 35-40°C and stir for 1 hour. After completely dissolving, cool down to 10-15°C. And at this temperature, add 10 g of the compound of formula II below, and keep it warm for 48 hours at the same temperature;
[0052] (2) step (1) completes the reaction, decompresses and evaporates hydrochloric acid to the utmost, obtains the compound of formula III; figure 1 It can be seen that the molecular weight of the product obtained in this step is consistent with the target product formula III compound;
[0053] (3) Add the compound of formula III obtained in step (2) into a 250 mL four-neck flask, and add 27 g of chloromethyl isopropyl carbonate, 30 mL of DMF, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com