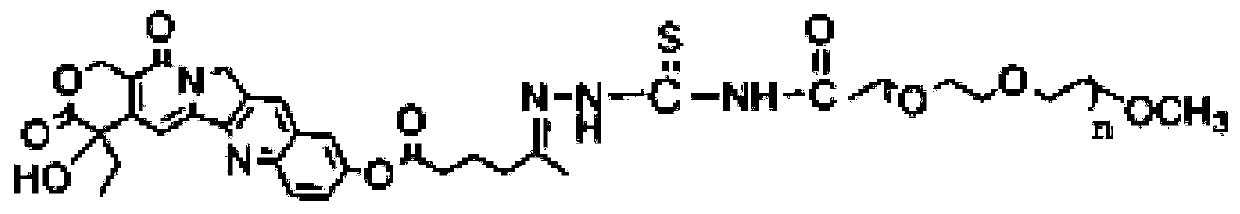10-Hydroxycamptothecin Water-Soluble Macromolecular Conjugates for pH Sensitive Release, Preparation Method and Application
A technology of water-soluble macromolecules and hydroxycamptothecin, which is applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as drug resistance and achieve drug loading High, no drug leakage, improved pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The technical solutions of the present invention will be clearly and completely described below in conjunction with the accompanying drawings. Apparently, the described embodiments are some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
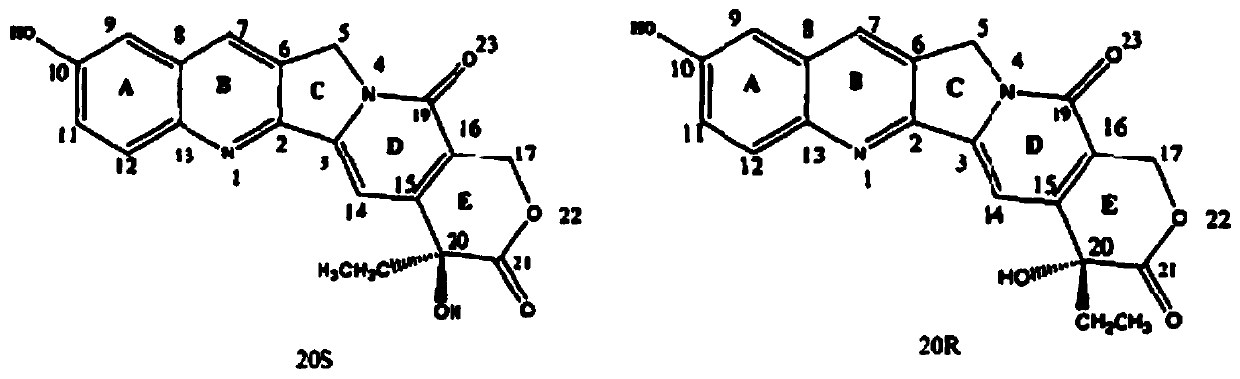
[0047] For the molecular structure of 10-hydroxycamptothecin (10-HCPT), see figure 1 , is composed of a pyrroloquinoline ring, a conjugated pyridine ring and a six-membered α-hydroxy lactone ring (ring E), molecular formula C 20 h 16 N 2 o 5 , molar mass 364.357, yellow prismatic monohydrate crystal, melting point 268-270°C, slightly soluble in a few organic solvents, insoluble in water, dissolved in the form of ring-opening carboxylate in dilute alkali solution, the solution has a blue color fluorescence....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com