Methods, reagents and cells for biosynthesizing compounds
A functionalization and conversion technology, applied in biochemical equipment and methods, organic chemistry, oxidoreductase, etc., can solve problems such as no reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
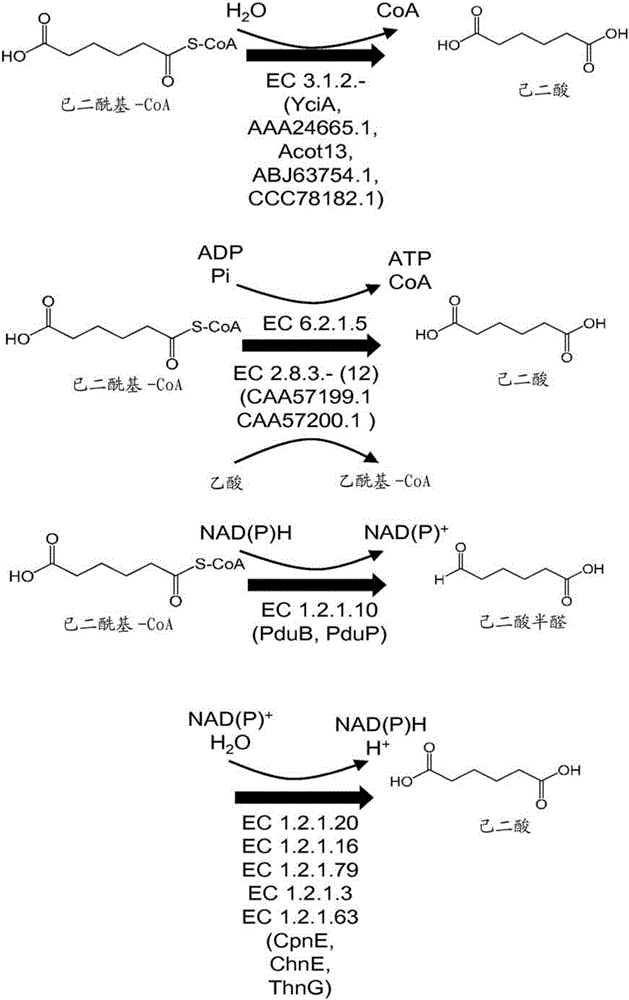
[0301] Enzyme activity of thioesterases using adipyl-CoA as substrate and forming adipate
[0302] The sequence encoding the N-terminal His tag was added to the gene from Escherichia coli encoding the thioesterase of SEQ ID NO:21 and SEQ ID NO:28 (see Figure 9 ), allowing the generation of N-terminal HIS-tagged thioesterases. Each resulting modified gene was cloned into the pET15b expression vector under the control of the T7 promoter. Each expression vector was transformed into a BL21[DE3] E. coli host. The resulting recombinant E. coli strain was cultured at 37° C. under shaking at 230 rpm in a 250 mL shake flask containing 50 mL of Luria Broth (LB) medium and antibiotic selection pressure. Cultures were induced overnight at 17°C with 0.5 mM IPTG.
[0303] Pellets from induced shake flask cultures were harvested via centrifugation. Resuspend each pellet, and in Y-per TM solution (ThermoScientific, Rockford, IL). Cell debris and supernatant were separated via centrifug...
Embodiment 2
[0306] Enzymatic activity of ω-transaminase using adipate semialdehyde as substrate and forming 6-aminocaproic acid
[0307] The nucleotide sequence encoding the His tag was added to the omega-transaminases encoding SEQ ID NOs: 13, 14, 15, 16, and 18 from Bacillus viridans, Pseudomonas aeruginosa, Pseudomonas syringae, spheroids Rhodobacter and Vibrio genes (see Figure 9 ), allowing the generation of N-terminally HIS-tagged ω-transaminases. Each resulting modified gene was cloned into a pET21a expression vector under the control of the T7 promoter, and each expression vector was transformed into a BL21[DE3] E. coli host. The resulting recombinant E. coli strains were grown in 250 mL shake flask cultures containing 50 mL LB medium and antibiotic selection pressure at 37° C. with shaking at 230 rpm. Each culture was induced overnight at 16°C with 1 mM IPTG.
[0308] Pellets from each induced shake flask culture were harvested via centrifugation. Each pellet was resuspended ...
Embodiment 3
[0314] Enzymatic activity of carboxylic acid reductases that use adipate as a substrate and form adipate semialdehyde
[0315] The nucleotide sequence encoding the HIS-tag was added to the genes from Segniliparus rugosus and Segniliparus rotundusta encoding the carboxylic acid reductases of SEQ ID NO: 9 and 12, respectively (see Figure 9 ), allowing the generation of an N-terminally HIS-tagged carboxylic acid reductase. Each modified gene was cloned into the pET Duet expression vector together with the sfp gene encoding the HIS-tagged phosphopantetheinyl transferase from Bacillus subtilis, both under the T7 promoter. Each expression vector was transformed into a BL21[DE3] E. coli host, and the resulting recombinant E. coli strains were grown in 250 mL shake flask cultures containing 50 mL LB medium and antibiotic selection pressure at 37°C, shaking at 230 rpm. Each culture was induced overnight at 37°C using autoinduction medium.
[0316] The pellet from each induced shake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com