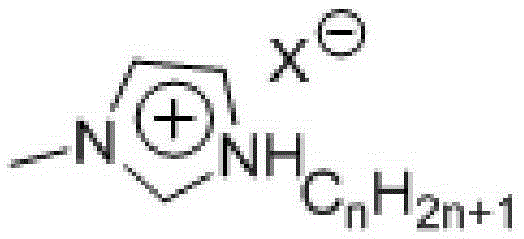Method for realizing continuous synthesis of 5-hydroxymethylfurfural through continuous extraction
A technology for extracting hydroxymethylfurfural, which is applied in the field of continuous synthesis of 5-hydroxymethylfurfural, can solve the problems of high single-pot yield, large reaction space, and inapplicability to large-scale production, and achieves improved yield and high efficiency. Effects of transformation and prolonging the usage period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
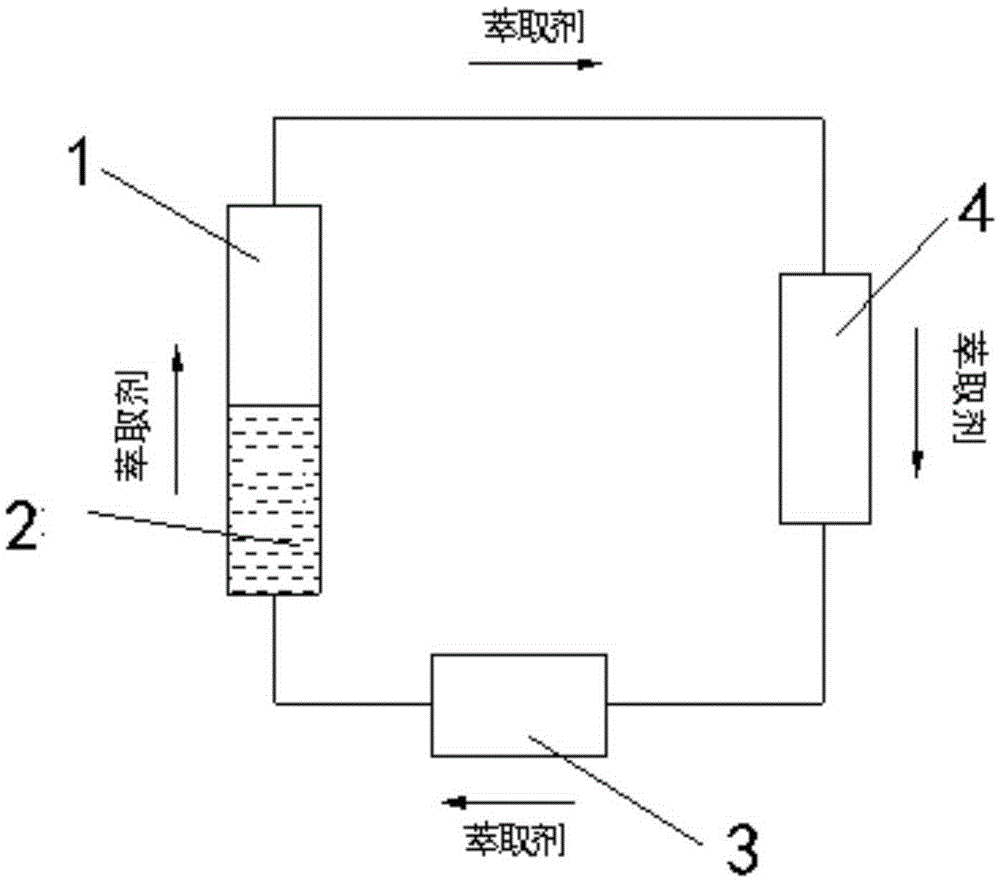
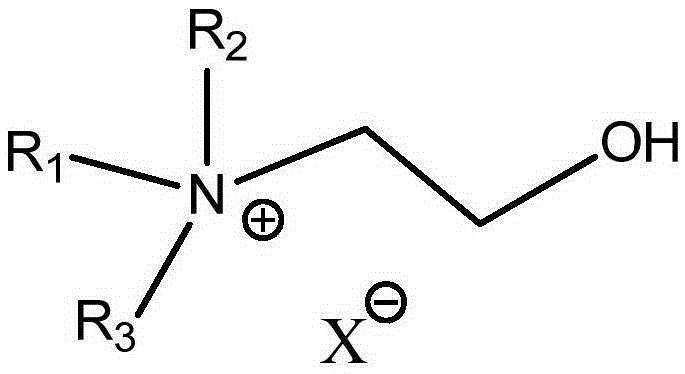
[0051] Using the above method, the reaction phase composed of 15g glucose, 30g ionic liquid (BMIMCl), 0.74g, and 6.0g water was reacted for 1 hour at 90°C and a pressure of 0.2Mpa, and ethylene glycol dimethyl ether was preheated to 90°C During the reaction process, ethylene glycol dimethyl ether continuously flows through the reaction phase at a speed of 10ml / min, and 5-hydroxymethylfurfural is continuously extracted out of the reaction system, and then flows into the separation system through the extraction agent quiet system for separation. The conversion rate of glucose was 26.4%, and the selectivity of 5-hydroxymethylfurfural was 70.5%.
Embodiment 2
[0053] Using the above method, 15g glucose, 30g ionic liquid (monobutyltrimethylimidazole chloride (BMIMCl)), 1.1g CrCl 3 -6H 2 O. The reaction phase composed of 6g water was reacted for 15 minutes at 130°C and pressure 2.0MP. During the reaction, ethylene glycol dimethyl ether was continuously preheated to 130°C and continuously transported at a speed of 20ml / min to enter the continuous reaction The device continuously flows through the reaction phase for extraction, and flows into the separation system through the extraction agent static system for separation. The conversion rate of glucose obtained was 97.6%, and the selectivity of 5‐hydroxymethylfurfural was 50.29%. Adopt the same proportioning, under the same reaction temperature and reaction time, in the batch tank reactor of small-scale magnetic force agitation, under the reaction condition that adds the extraction agent of reaction phase system 3 times, the conversion rate of glucose is only 66%, 5- The selectivity o...
Embodiment 3
[0055] Adopt above-mentioned method, will be made of 33gBMIMCl, 1.2g CrCl 3 -6H 2 The reaction phase composed of O, 6g water and 15g glucose was reacted for 10 minutes at 182°C and a pressure of 2.8Mpa. During the reaction, ethylene glycol dimethyl ether was preheated to 200°C and continued at a speed of 30ml / min. Flowing through the reaction phase, 5-hydroxymethylfurfural is extracted from the reaction phase, and flows into the separation system through the extraction agent static system for separation. The obtained glucose conversion rate is 96.9%, and the selectivity is 57.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com