Method for analyzing chiral drug enantiomers in biological body fluid through column-switching liquid chromatography
A liquid chromatography analysis and chiral drug technology, which is applied in the field of column switching-liquid chromatography analysis of chiral drug enantiomers in biological fluids, can solve the problems of time-consuming, solvent-consuming, large sample loss, etc., and achieves reduction of cumbersome steps, improve the degree of automation, and the effect of sensitive online detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 The method for column switching-liquid chromatography analysis of chiral drug enantiomers in biological fluids comprises the following steps:
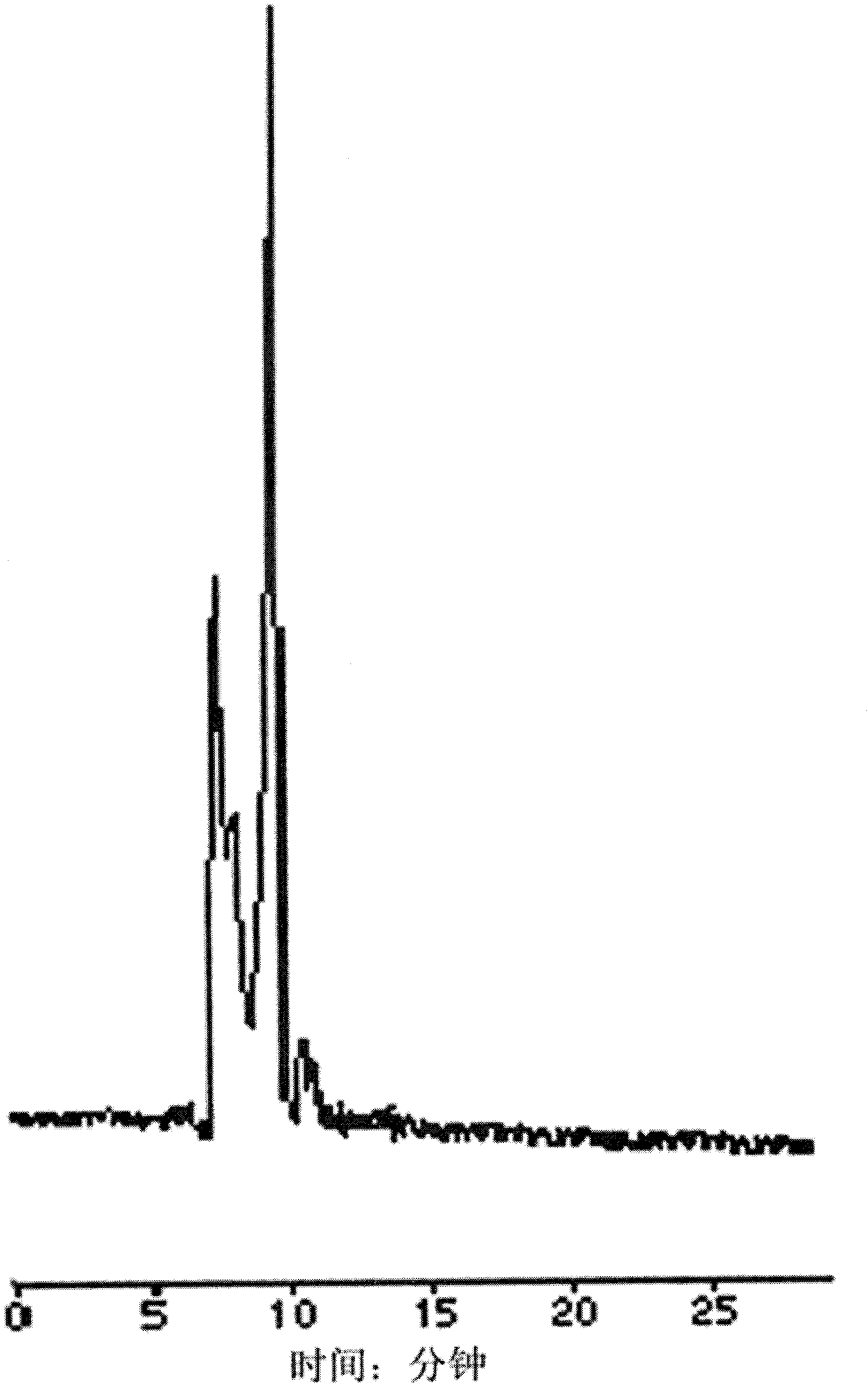
[0031] (1) Dissolve the standard substance of chiral drug racemate - propranolol hydrochloride racemate standard substance at a temperature of 20°C with absolute ethanol to make a standard solution with a concentration of 5mg / mL; the standard solution Use blank plasma at a temperature of 20°C (see figure 1 ) was diluted to make a plasma sample with a concentration of 0.5 mg / mL.
[0032] (2) Put the plasma sample in a 1.5mL centrifuge tube with a stopper, centrifuge at a speed of 4000r / min for 5min, carefully draw the supernatant and place it in a centrifuge tube for sample injection.
[0033] (3) Preparation of limited-access packing column: the outer surface of silica gel with a particle size of 5 μm is bonded with hydrophilic polyvinyl alcohol, and the inner surface is bonded with non-polar hexylamine to form a colu...
Embodiment 2
[0041] Example 2 The method for column switching-liquid chromatography analysis of chiral drug enantiomers in biological fluids, comprising the following steps:
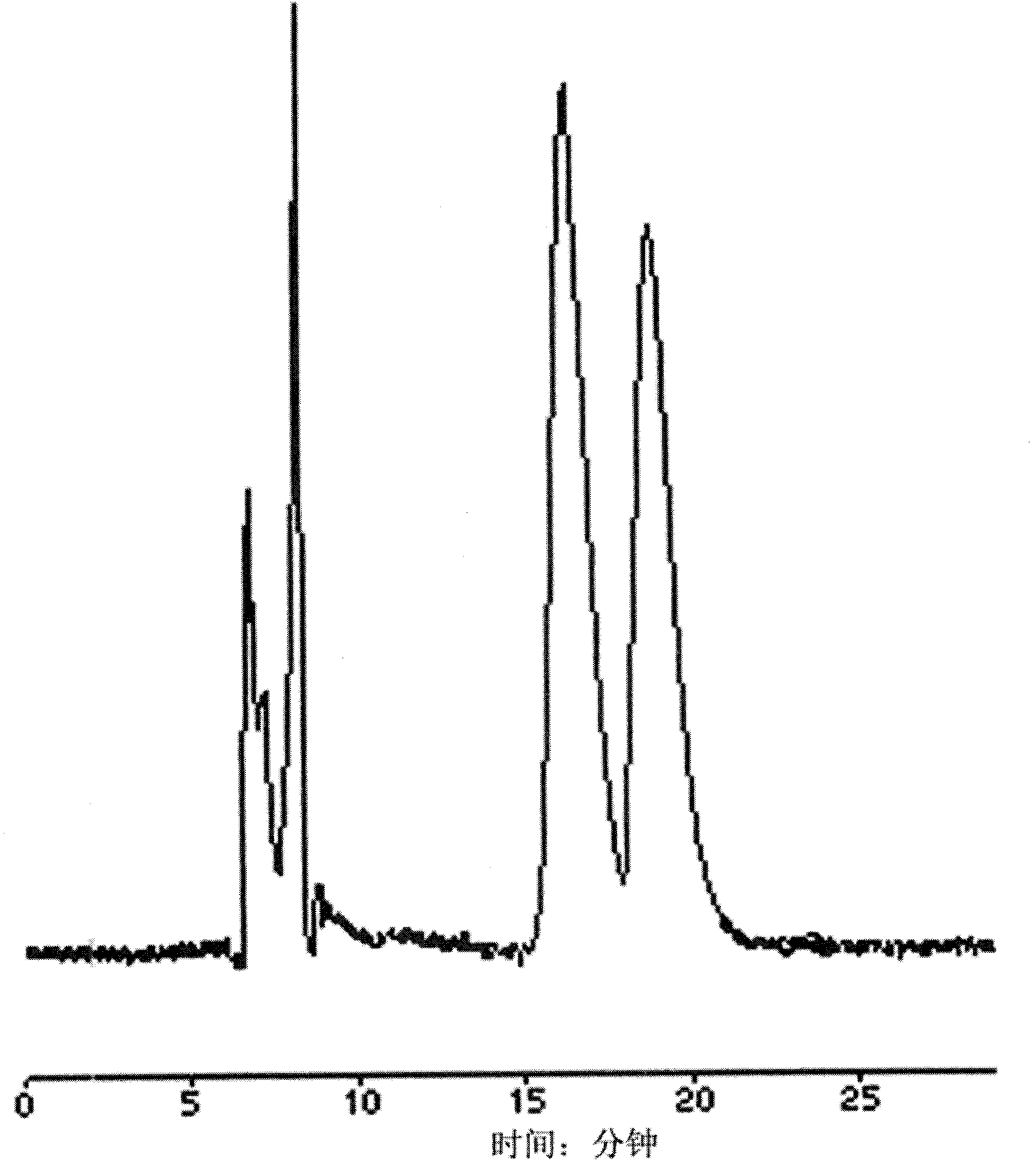
[0042] (1) Dissolve the standard substance of chiral drug racemate - the standard substance of propranolol hydrochloride racemate at a temperature of 30°C with absolute ethanol to make a standard solution with a concentration of 4mg / mL; the standard solution Use blank plasma at a temperature of 30°C (see image 3 ) was diluted to make a plasma sample with a concentration of 1.5 mg / mL.
[0043] (2) Put the plasma sample in a 2.0mL centrifuge tube with a stopper, centrifuge at a speed of 2000r / min for 3min, carefully draw the supernatant and place it in a centrifuge tube for sample injection.
[0044] (3) Preparation of limited-access packing column: the outer surface of silica gel with a particle size of 5 μm is bonded with hydrophilic polyvinyl alcohol, and the inner surface is bonded with non-polar hexylamine to fo...
Embodiment 3
[0052] Example 3 A method for column switching-liquid chromatography analysis of chiral drug enantiomers in biological fluids, comprising the following steps:
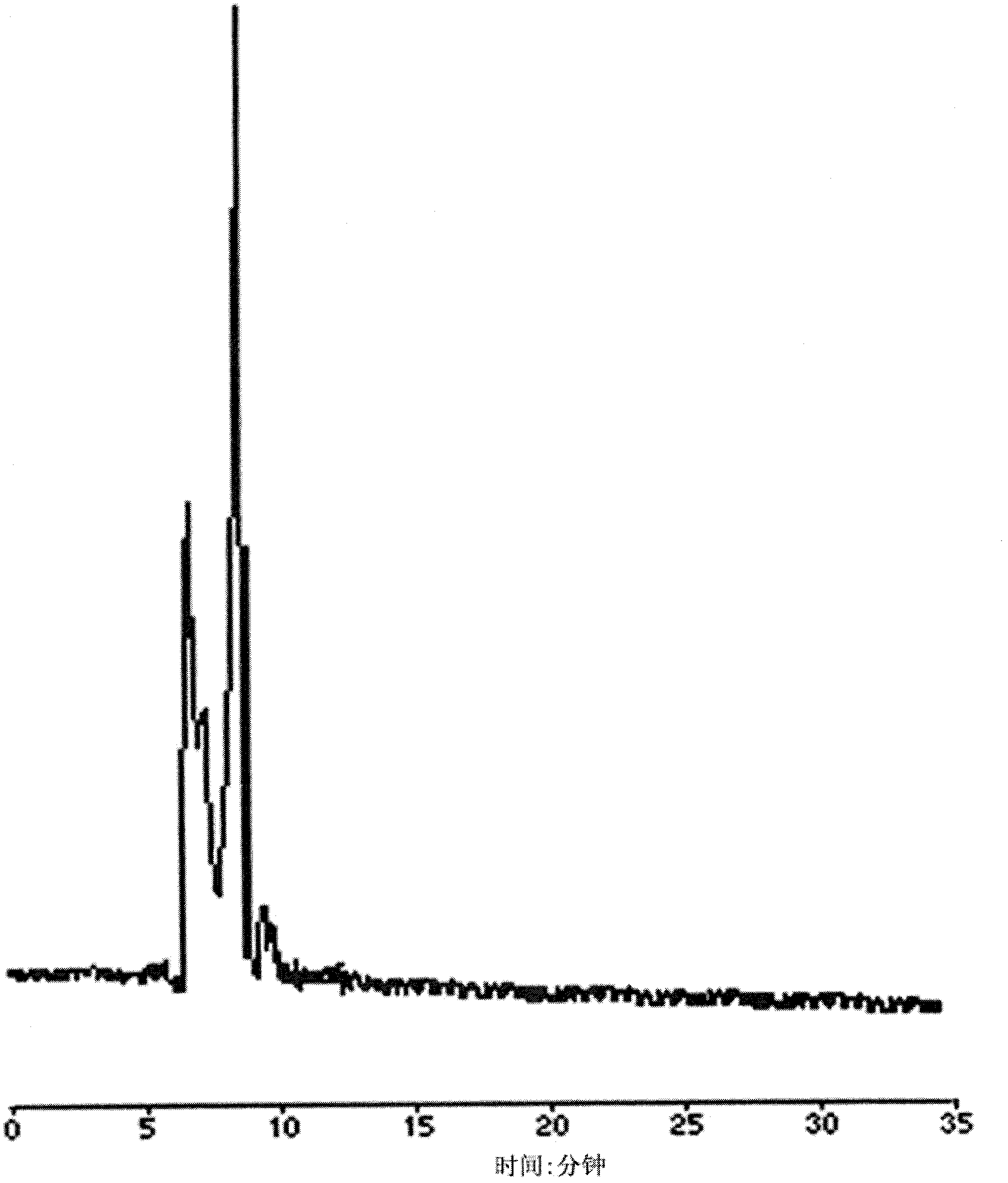
[0053] (1) Dissolve the standard substance of chiral drug racemate - propranolol hydrochloride racemate standard substance with absolute ethanol at a temperature of 5°C to make a standard solution with a concentration of 4.5mg / mL; The solution was diluted with blank plasma at a temperature of 5°C to prepare a plasma sample with a concentration of 1.0 mg / mL.
[0054] (2) Put the plasma sample in a 1mL centrifuge tube with a stopper, centrifuge at a speed of 1000r / min for 10min, carefully draw the supernatant and place it in a centrifuge tube for sample injection.
[0055] (3) Preparation of limited-access packing column: the outer surface of silica gel with a particle size of 5 μm is bonded with hydrophilic polyvinyl alcohol, and the inner surface is bonded with non-polar hexylamine to form a column with an inner diamet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com