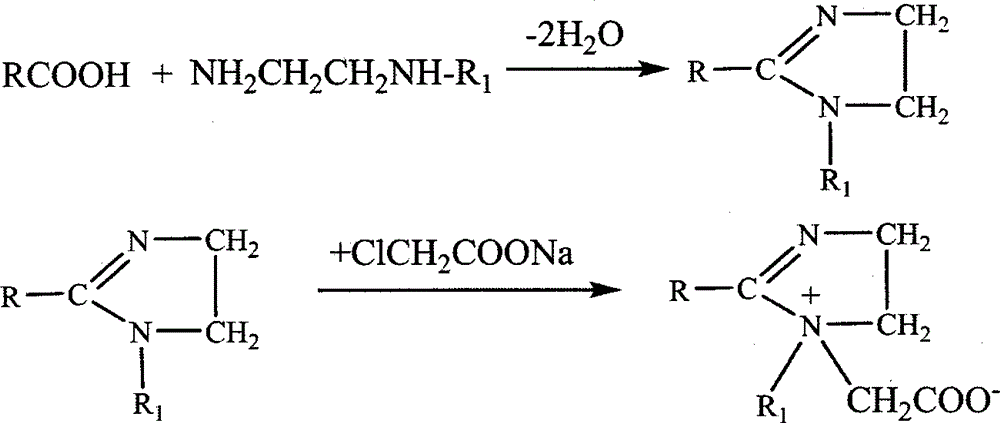
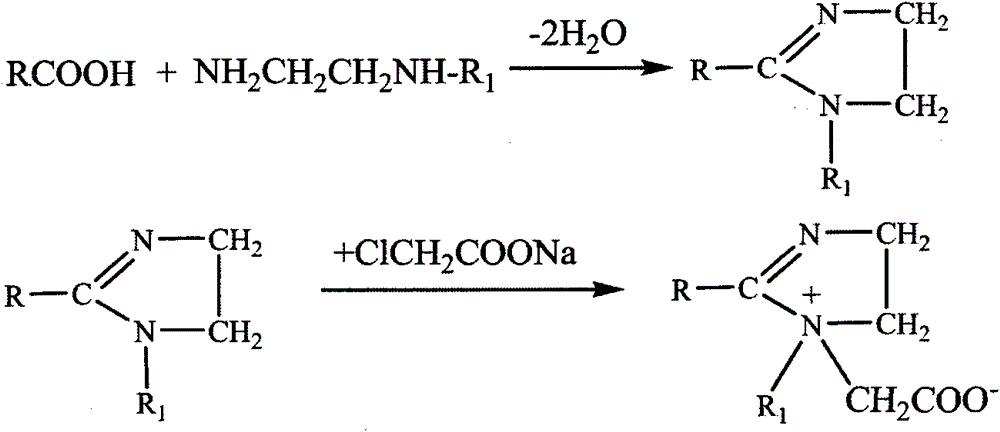
Synthesis method for imidazoline ampholytic surfactant
A technology of surfactant and synthesis method, applied in chemical instruments and methods, dissolution, organic chemistry, etc., can solve the problems of high energy consumption, difficult recycling, easy oxidation of amines, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The synthetic method of embodiment 1. imidazoline laurate amphoteric surfactant
[0014] Step 1: in the 500mL four-necked flask that magnetic stirring, thermometer and decompression distillation device are housed, add a certain amount of lauric acid and diethylenetriamine, make the mol ratio of lauric acid and diethylenetriamine be 1: 1.2, Under nitrogen protection, start heating and stir slowly. When the temperature is around 44°C, lauric acid begins to melt. When the temperature rises to about 72°C, lauric acid and diethylenetriamine are completely mixed, and then the reaction temperature is raised to 130°C. At about ℃, water vapor began to appear on the bottle wall at this time. Start the vacuum pump to adjust the vacuum degree of the reaction system to 0.07MPa, and continue to heat up. When the temperature is 140℃, the first drop of water appears in the receiver, and the reaction continues to form water. When the temperature rises to 160°C, the water outlet speed be...
Embodiment 2
[0016] The synthetic method of embodiment 2. imidazoline stearic acid amphoteric surfactant
[0017] Step 1: In the 500mL four-neck flask that magnetic stirring, thermometer and vacuum distillation device are housed, add a certain amount of stearic acid and hydroxyethylethylenediamine, make the mole of stearic acid and hydroxyethylethylenediamine The ratio is 1:1.2, nitrogen protection, start heating and stirring slowly, when the reaction temperature rises to about 130°C, water vapor begins to appear on the bottle wall at this time, start the vacuum pump to adjust the vacuum degree of the reaction system at 0.08MPa, continue to heat up, When the temperature is 140°C, the first drop of water appears in the receiver, and the reaction continues to form water. When the temperature rises to 160°C, the water outlet speed becomes slower, and almost no water is formed. The reaction time at this stage is about 2 hours. Continue to heat up. When the temperature is about 200°C, the amoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com