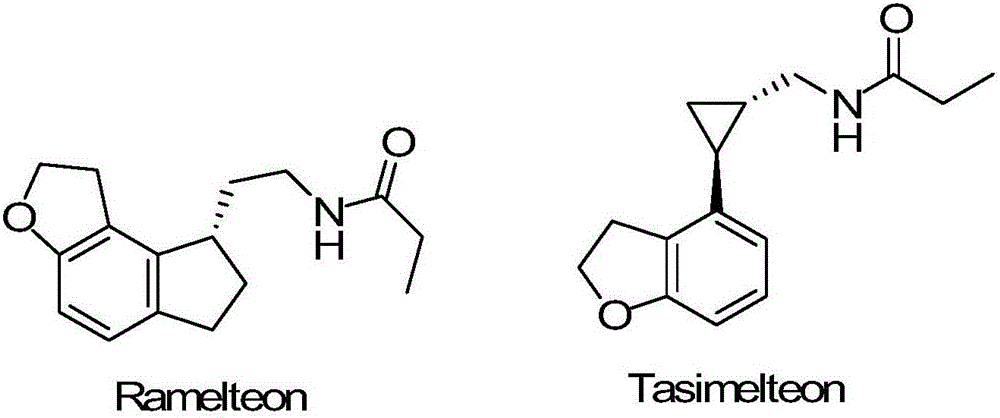
Method for preparing 2,3-dihydrobenzofuran formaldehyde
A technology of furan formaldehyde and dihydroxybenzaldehyde, applied in the direction of organic chemistry and the like, can solve problems such as complex synthesis process and harsh conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A method for preparing 2,3-dihydrobenzofuran carbaldehyde, characterized in that it comprises the steps of:
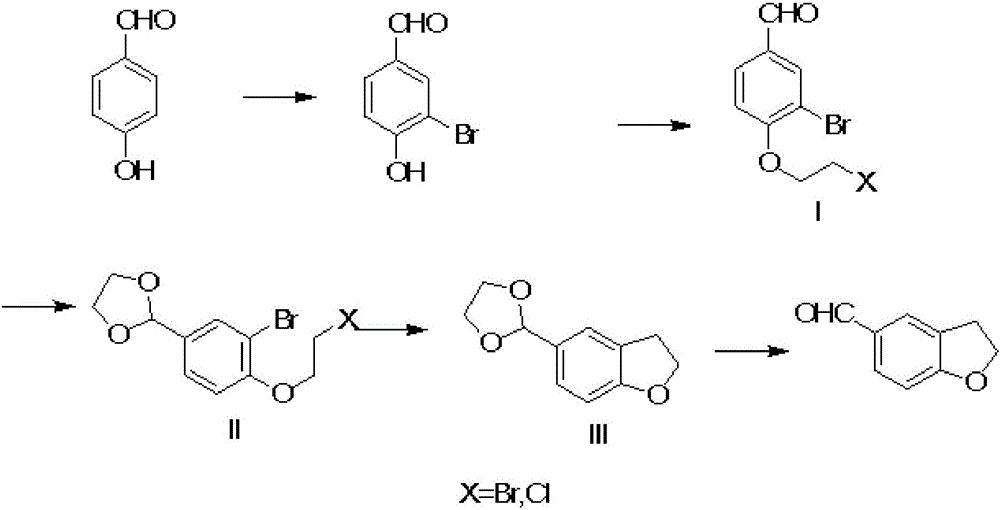
[0024] 1), take p-Hydroxybenzaldehyde as raw material, obtain 3-bromo-4-hydroxyl-benzaldehyde after bromination,
[0025] 2), docking 3-bromo-4-hydroxy-benzaldehyde with 1-bromo-2 chloroethane under the action of organic solvent DMF and potassium carbonate to obtain intermediate I, the reaction temperature is 40°C-50°C,
[0026] 3), the aldehyde group of the above-mentioned intermediate I reacts with ethylene glycol in the organic solvent toluene under the catalysis of p-toluenesulfonic acid to form the acetal intermediate II, and the reaction temperature is 90-100°C;
[0027] 4), Intermediate II uses methyl THF as a solvent, and undergoes self-cyclization under the action of magnesium strips to form 2,3-dihydrobenzofuran intermediate III. Reaction temperature: 40-50°C;
[0028] 5) The acetal protection of intermediate III is removed under acidic conditions to ...
Embodiment 2
[0032] A method for preparing 2,3-dihydrobenzofuran carbaldehyde, characterized in that it comprises the steps of:
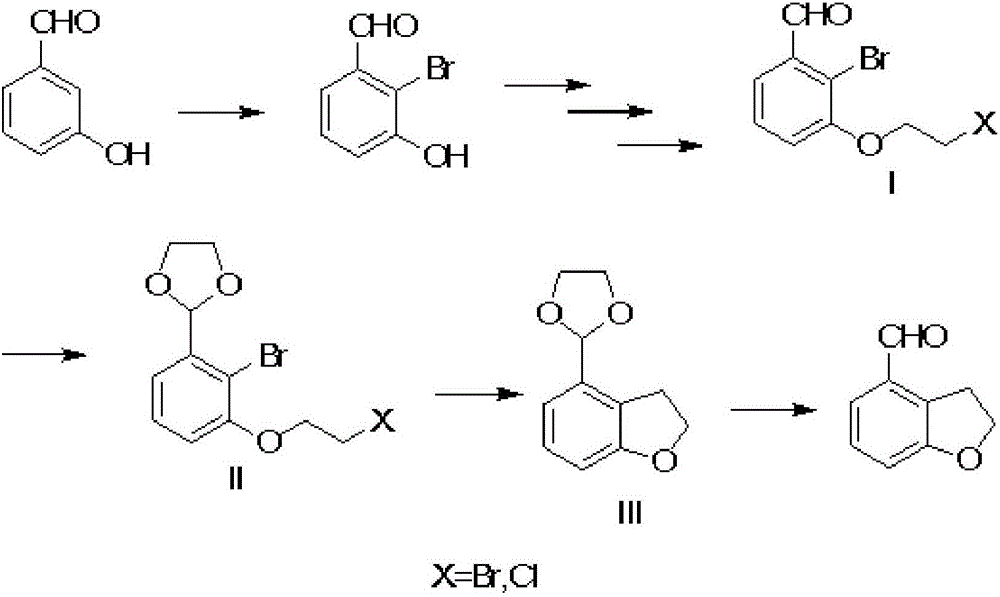
[0033] 1), take m-hydroxybenzaldehyde as raw material, obtain 2-bromo-3-hydroxyl-benzaldehyde after bromination,
[0034] 2), docking 2-bromo-3-hydroxy-benzaldehyde with 1,2-dibromoethane under the action of organic solvent acetonitrile and sodium carbonate to obtain intermediate I, the reaction temperature is 65°C-80°C,
[0035] 3), the aldehyde group of the above-mentioned intermediate I reacts with ethylene glycol in the organic solvent xylene under the catalysis of sulfuric acid to form the acetal intermediate II, and the reaction temperature is 110-125°C;
[0036] 4) Intermediate II uses diethyl ether and THF as solvents, and undergoes self-cyclization under the action of butyllithium to form 2,3-dihydrobenzofuran intermediate III. Reaction temperature: -20~0°C;
[0037] 5) The acetal protection of intermediate III is removed under the acidic condition of ...
Embodiment 3
[0041] A method for preparing 2,3-dihydrobenzofuran carbaldehyde, characterized in that it comprises the steps of:
[0042] 1), take p-Hydroxybenzaldehyde as raw material, obtain 3-bromo-4-hydroxyl-benzaldehyde after bromination,
[0043] 2), under the action of organic solvent DMF and potassium carbonate, 3-bromo-4-hydroxy-benzaldehyde is docked with bromoethane, and then brominated to obtain intermediate I, the reaction temperature is 80°C-90°C,
[0044] 3), the aldehyde group of the above-mentioned intermediate I reacts with ethylene glycol in the organic solvent toluene and xylene under the catalysis of p-toluenesulfonic acid to form the acetal intermediate II, and the reaction temperature is 130-140°C;
[0045] 4), Intermediate II uses methyl THF as a solvent, cyclizes itself under the action of magnesium strips to form 2,3-dihydrobenzofuran intermediate III, reaction temperature: 60-70°C;
[0046] 5) The acetal protection of intermediate III is removed under acidic cond...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com