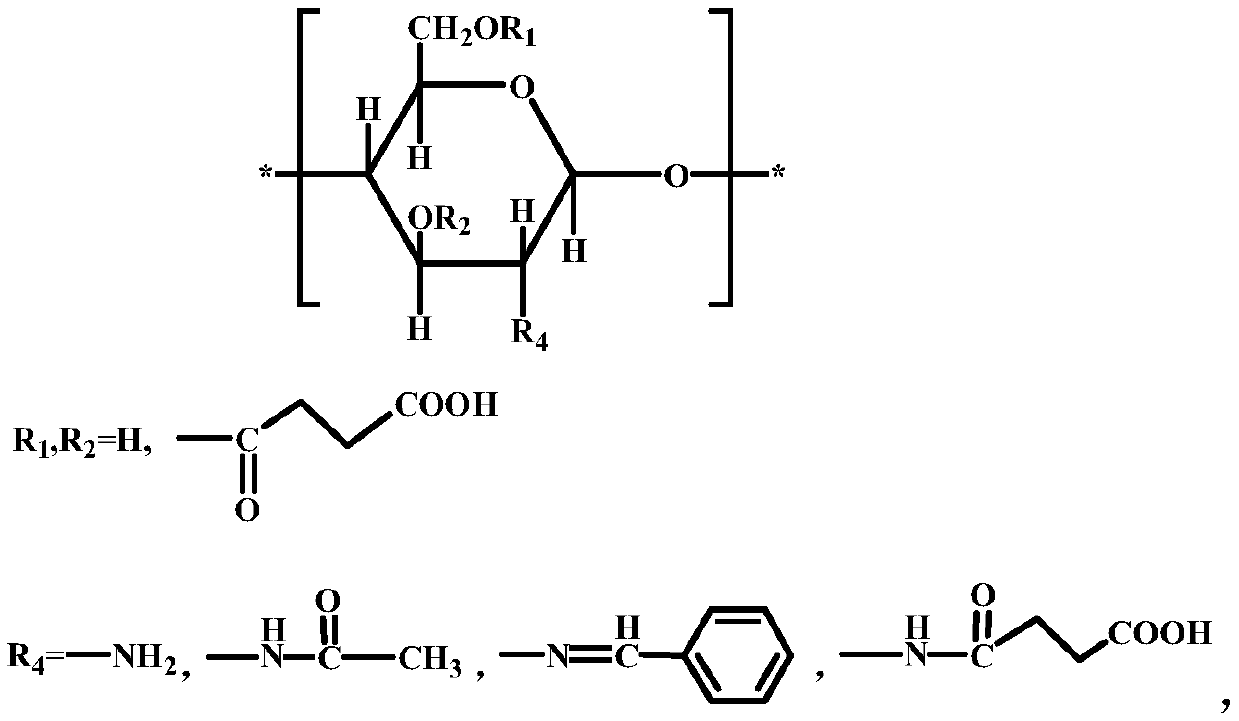
A kind of O-succinate chitosan Schiff base and preparation method thereof
A kind of technology of succinic acid chitosan mat and succinic acid chitosan, applied in the preparation of above-mentioned O-succinic acid chitosan Schiff base, O-succinic acid chitosan Schiff base field, can Solve the problems of more synthesis and less research, and achieve the effect of improving water solubility, good corrosion inhibition, and enriching functional active groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
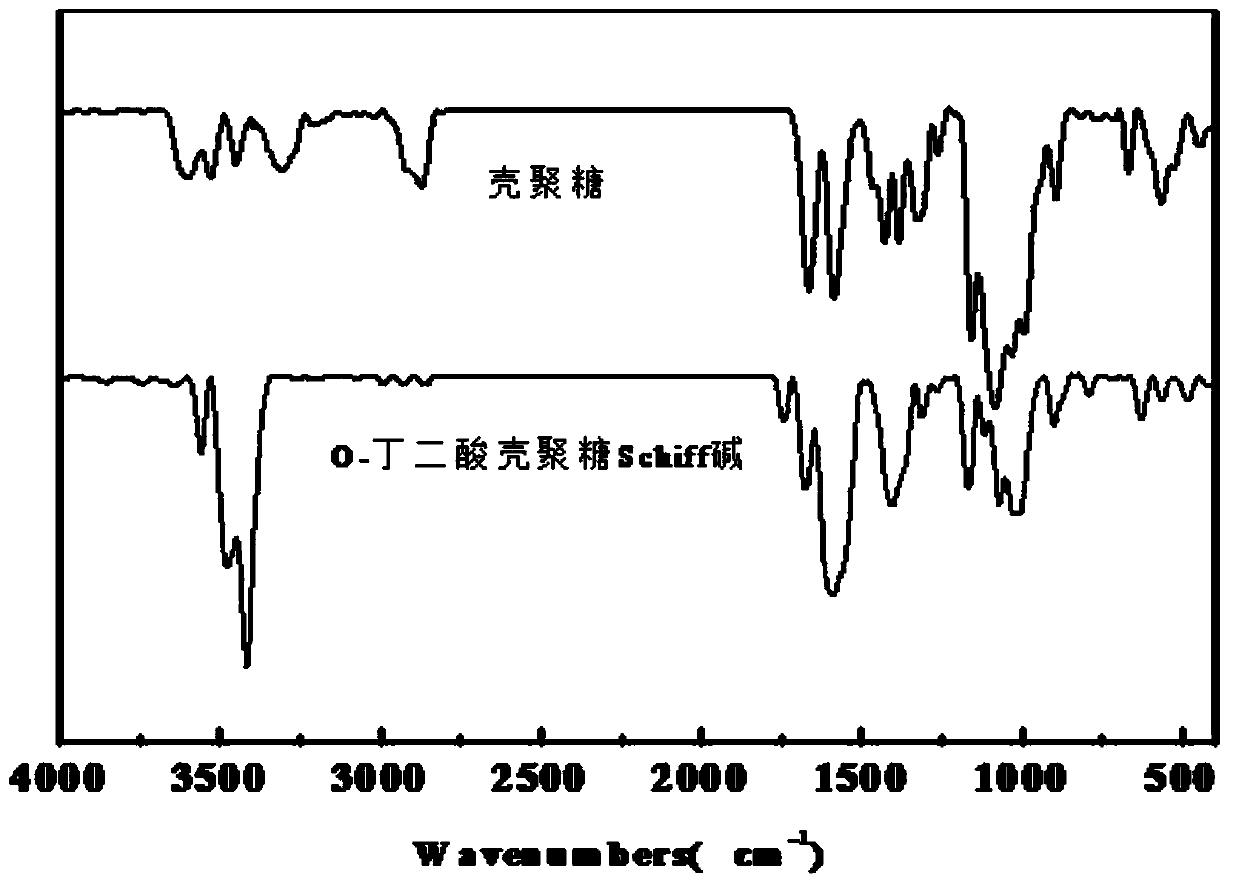
Image
Examples
preparation example Construction
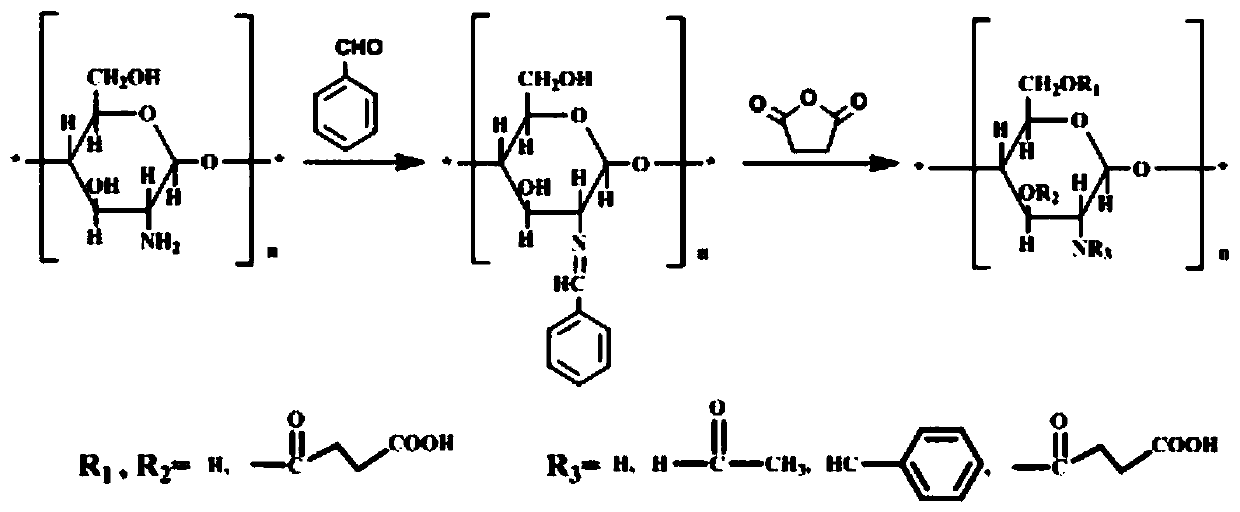
[0031] The present invention also provides a preparation method of the above-mentioned O-succinic acid chitosan Schiff base, which comprises the following steps:
[0032] Step 1. Prepare benzaldehyde chitosan Schiff base. The specific process is: Step 1.1, take 1 part of chitosan, the molecular weight of chitosan is 700,000, the degree of deacetylation is 87%, and it is dissolved in 1% In wt acetic acid solution, the pH of the solution was adjusted to 7 with 1mol / L sodium hydroxide, filtered with suction and washed with ethanol to obtain a filter cake; step 1.2, add the filter cake to the ethanol solution at 70℃ , Use 1mol / L hydrogen chloride or 1mol / L sodium hydroxide solution to adjust its pH to 6, and then add 1.3 parts of benzaldehyde dissolved in ethanol solution, after reaction for 4h, suction filtration and washing with ethanol to obtain benzaldehyde shell poly Sugar Schiff base.
[0033] Step 2. Take 0.5 parts by mass of benzaldehyde chitosan Schiff base, mix it with 30 pa...
Embodiment 1
[0038] The specific process of preparing O-succinic acid chitosan Schiff base is:
[0039] Step 1. Prepare benzaldehyde chitosan Schiff base. The specific process is: Step 1.1, take 1g of chitosan, the molecular weight of chitosan is 700,000, and the degree of deacetylation is 87%. Dissolve it in 50ml. In a 1% wt acetic acid solution, stir at room temperature for 20 minutes, then use 1 mol / L sodium hydroxide to adjust the pH of the solution to 7, suction filtration and wash with ethanol to obtain a filter cake; step 1.2, add ethanol to the filter cake In the solution, use 1mol / L hydrogen chloride to adjust its pH to 6 at 70℃, then add dropwise 20ml ethanol solution with 1.3g benzaldehyde dissolved in it, react for 4h, filter with suction and wash with ethanol to obtain the benzaldehyde shell Glycan Schiff base.
[0040] Step 2, 0.5g of benzaldehyde chitosan Schiff base obtained in step, mix it with 30ml dichloromethane solution and 1ml acid binding agent solution, the acid binding...
Embodiment 2
[0045] The specific process of preparing O-succinic acid chitosan Schiff base is:
[0046] Step 1. Prepare benzaldehyde chitosan Schiff base. The specific process is: Step 1.1, take 1g of chitosan, the molecular weight of chitosan is 700,000, and the degree of deacetylation is 87%. Dissolve it in 50ml. In a 1% wt acetic acid solution, stir at room temperature for 20 minutes, then use 1 mol / L sodium hydroxide to adjust the pH of the solution to 7, suction filter and wash with ethanol to obtain a filter cake; step 1.2, add the filter cake to 40 ml In the ethanol solution, use 1 mol / L hydrogen chloride or 1 mol / L hydrogen chloride solution to adjust its pH to 6 at 70°C, then add dropwise 20 ml of ethanol solution in which 1.3 g benzaldehyde is dissolved, and after reacting for 4 hours, after suction filtration Wash with ethanol to obtain benzaldehyde chitosan Schiff base.
[0047] Step 2, 0.5g of benzaldehyde chitosan Schiff base obtained in step, mix it with 30ml of dichloromethane ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com