High-risk human papilloma virus kit and detection method
A technology of human papillomavirus and detection method, applied in the direction of microorganism-based method, biochemical equipment and method, microorganism measurement/testing, etc., can solve the problems of increasing HPV subtypes, linear increase of detection cost, etc. Uniformity, extended annealing time, beneficial effects of binding and extension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The kit of the present invention comprises multiplex PCR primers and fluorescent labeling primers as shown in Table 1 and Table 2
[0065]
[0066]
[0067]
[0068] Table 1
[0069]
[0070] Table 2
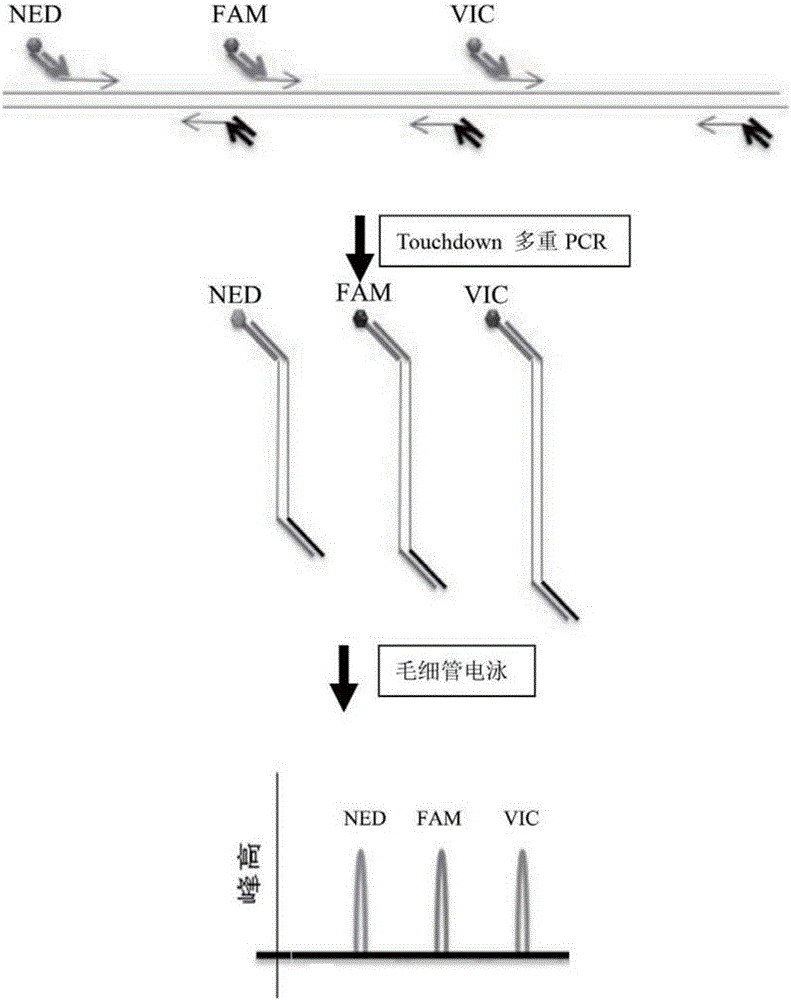
[0071] Table 1 includes the sequence information and concentration of the multiplex PCR primers, and Table 2 is the information table of the fluorescently labeled primers.
Embodiment 2
[0073] 1. Multiplex PCR reaction
[0074] Reaction system 10uL: DDH2O 2.35uL, 10*PCR Buffer 1uL, 25nM Mg2+1uL, 2nM DNTP1.5uL, adapter primer mixture (see Table 1 for primer information) 1uL, fluorescent labeling primer mixture (primer information see Table 2) 1uL, 5U / uL Faststart Taq enzyme 0.15uL, HPV16 positive sample DNA 2uL. PCR cycle program: 95°C 4min; 11cycles x (94°C 30s, 68°C-0.5°C / cycle 120s); 36cycles x (94°C 30s, 58°C 60s); 72°C 10min; 4°C for ever.
[0075] 2. Genetic analyzer on the PCR product
[0076] Take 1uL of the PCR product and dilute it 50 times; take 1uL of the diluted solution, mix it with 0.06μl Liz120SIZE STANDARD and 8.9μl Hi-Di, and perform capillary electrophoresis and genotype analysis on a genetic analyzer ABI3130. HPV subtypes are determined based on peaks of specific color and position on the capillary electrophoresis map.
[0077] 3. Result Analysis
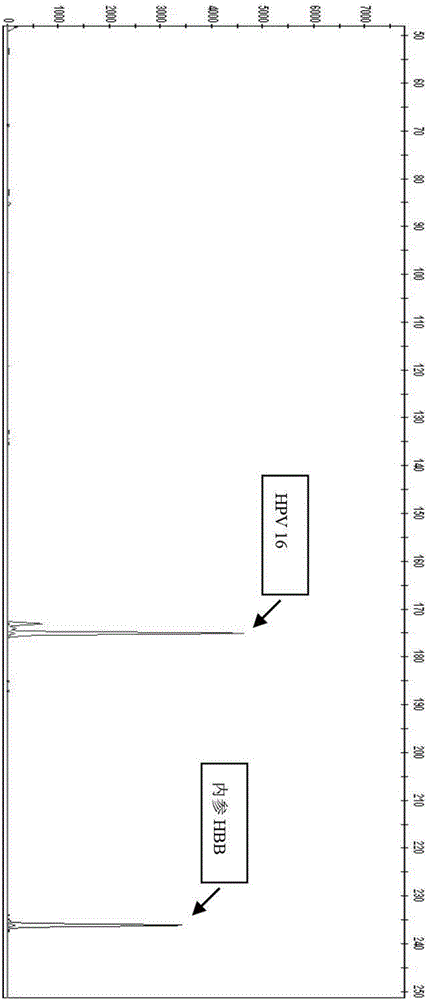
[0078] Test results such as image 3 Shown as HPV16 positive.
[0079] When using the s...
Embodiment 3
[0081] 1. Multiplex PCR reaction
[0082] Reaction system 10uL: DDH2O 2.35uL, 10*PCR Buffer 1uL, 25nM Mg2+1uL, 2nM DNTP1.5uL, adapter primer mixture (see Table 1 for primer information) 1uL, fluorescent labeling primer mixture (primer information see Table 2) 1uL, 5U / uL Faststart Taq enzyme 0.15uL, HPV52 positive sample DNA 2uL. PCR cycle program: 95°C 4min; 11cycles x (94°C 30s, 68°C-0.5°C / cycle 120s); 36cycles x (94°C 30s, 58°C 60s); 72°C 10min; 4°C for ever.
[0083] 2. Genetic analyzer on the PCR product
[0084] Take 1uL of the PCR product and dilute it 50 times; take 1uL of the diluted solution, mix it with 0.06μl Liz120SIZE STANDARD and 8.9μl Hi-Di, and perform capillary electrophoresis and genotype analysis on a genetic analyzer ABI3130. HPV subtypes are determined based on peaks of specific color and position on the capillary electrophoresis pattern.
[0085] 3. Result Analysis
[0086] Test results such as Figure 5 Shown as HPV16 positive.
[0087] When using ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com