Applications and method of quinoline substance in treating thyroid and mammary gland diseases, and medicinal composition
A breast disease and composition technology, applied in the field of pharmaceutical compositions containing quinoline substances, can solve problems such as unsatisfactory curative effect and immune system damage, and achieve friendly body disturbance, good curative effect, and high compatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
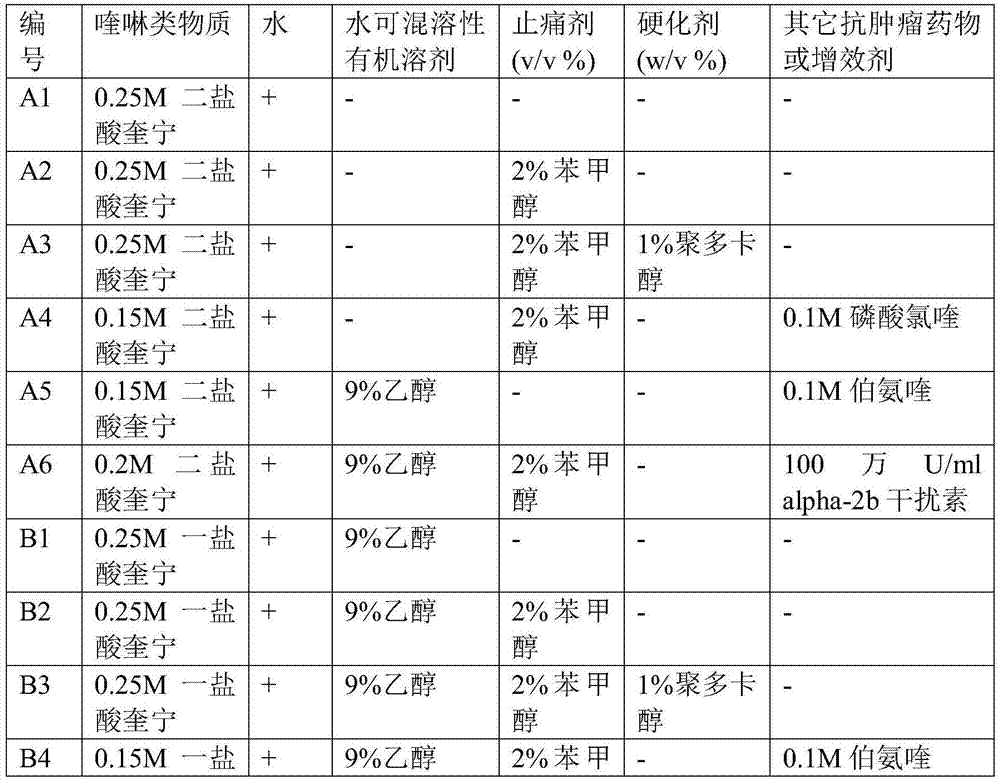
[0058] Embodiment 1: the preparation of quinoline substance injection
[0059] Table 1
[0060]
[0061]
[0062] Remarks: + refers to water for injection except for the composition in the table;
[0063] -Refers to does not contain.
[0064] In Table 1, the preparation method of A2 is as follows: at room temperature, add 0.2ml of benzyl alcohol to 8.5ml of water for injection to dissolve, then add 758mg of quinine dihydrochloride dry powder to the solution for dissolution, then add water for injection to make the total volume reach 10ml , mix evenly and then pack into 2ml / bottle for later use, so as to prepare injection A2.
[0065]The preparation method of A3 is as follows: at room temperature, add 0.2ml of benzyl alcohol into 8.0ml of water for injection to dissolve, then add 758mg of quinine dihydrochloride dry powder into the solution to dissolve, then add 1000mg of polidocanol to dissolve, and finally add water for injection Make the total volume reach 10ml, aft...
Embodiment 2
[0071] Embodiment 2: the optimal condition research of application
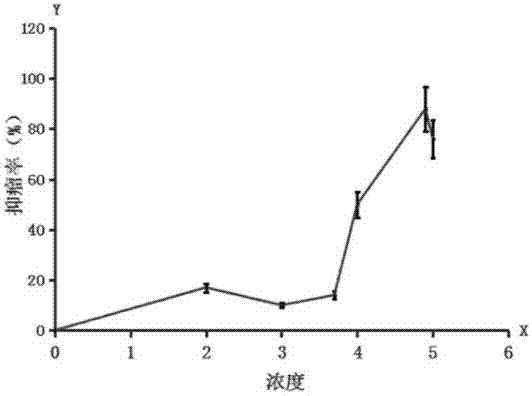
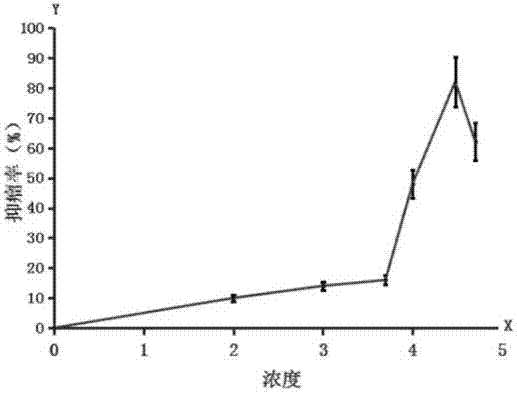
[0072] The preferred conditions for the application of the quinoline substances of the present invention in the treatment of thyroid and breast diseases were firstly studied in anti-tumor experiments (in vitro anti-cancer cell experiments and transplanted tumor animal model experiments).
[0073] In antitumor experiments, positive controls are usually existing clinical drugs (such as cisplatin, doxorubicin, mitomycin, gemcitabine, 5-fluoropyrimidine, etc.). Their clinical significance lies in low toxicity and high activity, such as low toxicity and up to 75% tumor inhibition rate in animal experiments. The term "anti-tumor activity", sometimes referred to as activity, refers to the property of having certain anti-tumor similarities compared with existing clinical drugs. A substance has a lower half-inhibitory concentration (abbreviated as IC) in vitro experiments 50 , usually less than 50 μM in the 72-hour ...
Embodiment 3
[0105] Embodiment 3: application in the treatment of breast disease
[0106] Clinically, the most common breast disease is breast enlargement, including tumor and non-neoplastic enlargement. In this example, the research on the application of quinolines was first carried out using quinine dihydrochloride. The preparation method of the injection of each medicine research group (B, C, D, E, F group) is identical with the preparation method of A2 in the table 1, contains 2% benzyl alcohol, but contains required different concentrations of quinine dihydrochloride, The injection concentrations are 0.025M, 0.05M, 0.15M, 0.25M, 0.4M, respectively. The injection dose is less than 300 mg quinine dihydrochloride / kg animal, and the injection volume is determined by the injection dose and concentration.
[0107] 1. Breast tumor treatment
[0108] The experimental animals were nude mice bearing breast cancer cells, and were divided into a negative control group, a positive control group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com