Metal organic skeleton material for removing cobalt ions from wastewater and preparation method of metal organic skeleton material
A metal organic framework, cobalt ion technology, used in the preparation of organic compounds, organic chemistry, cyanide reaction preparation and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
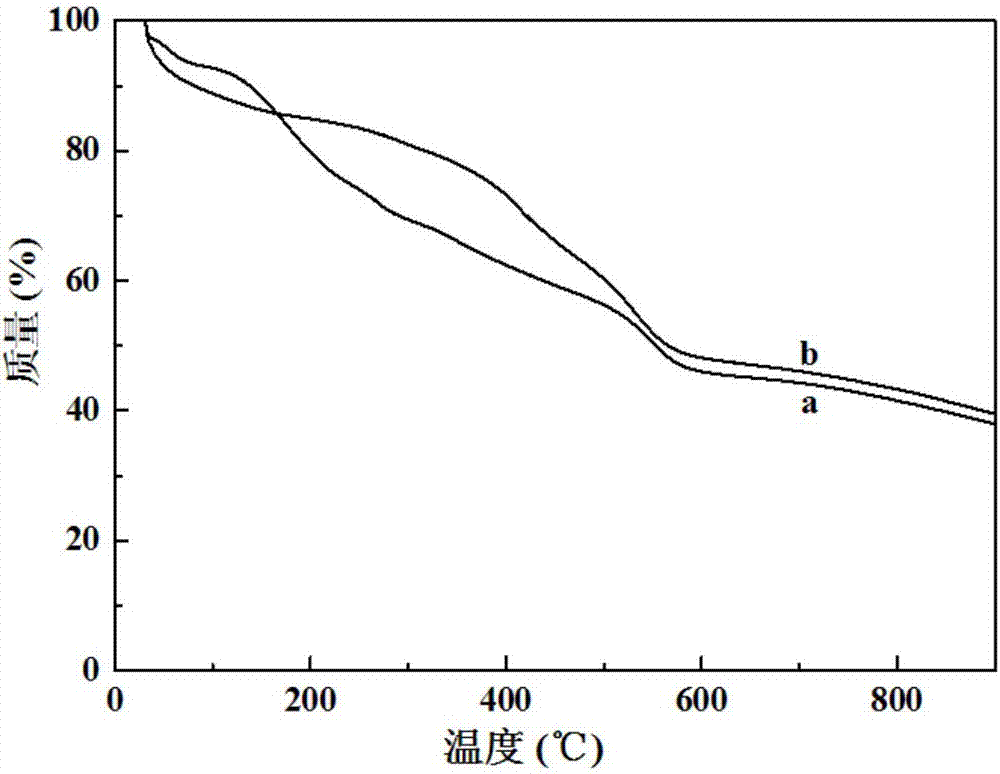
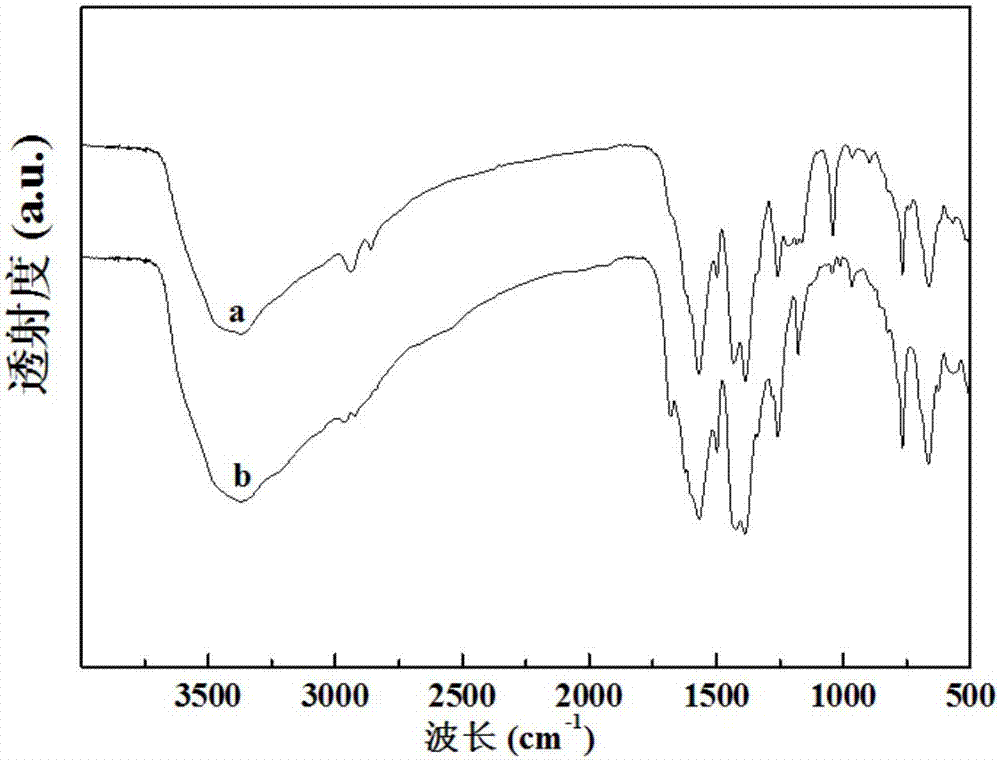
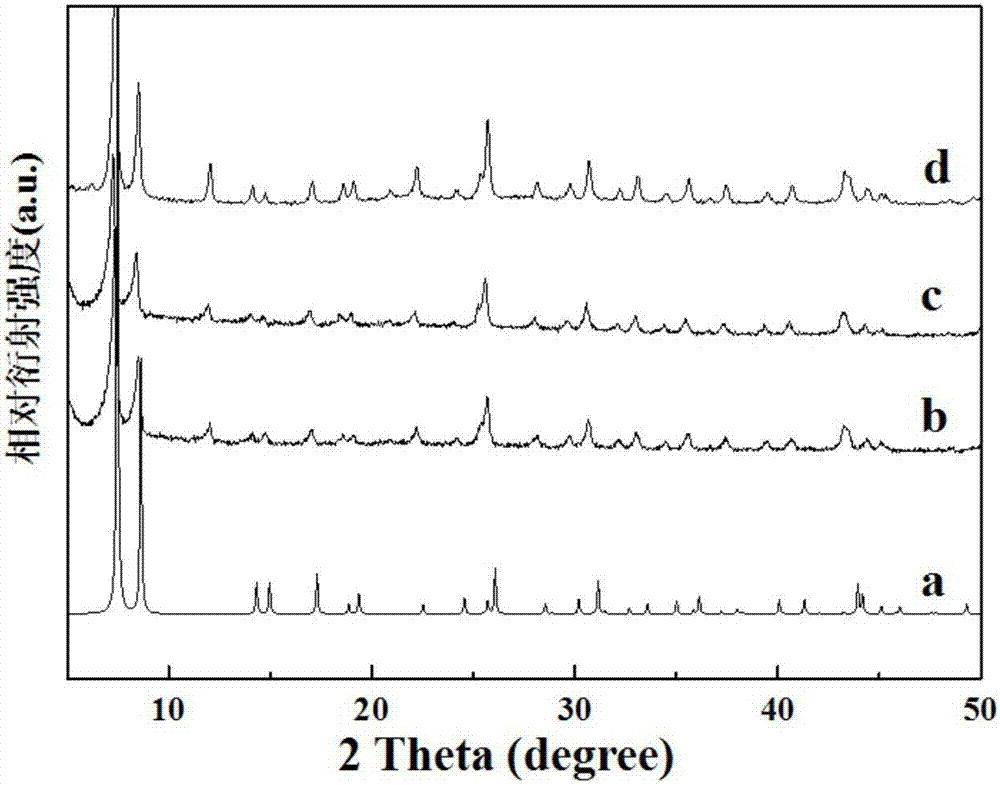
[0041] Take 1.34g of 2-aminoterephthalic acid and 1.25g of zirconium tetrachloride, dissolve them together in 150ml of N,N-dimethylformamide; add 5.0ml of hydrochloric acid, stir well and then sonicate for 15 minutes , and then the mixed solution was transferred to a hydrothermal reaction kettle, heated to 120°C, and kept at this temperature for 48 hours; naturally cooled to room temperature, filtered, washed with ethanol three times, and dried to obtain a white solid powder UiO-66 -NH 2 ; The resulting white solid powder UiO-66-NH 2 Take 2.0 g and disperse it in 100 ml of ethanol, add 1.0 g of terephthalaldehyde to obtain a mixed solution, reflux the mixed solution for 10 hours, cool to room temperature, filter, wash with ethanol three times, and dry to obtain a yellow solid powder UiO -66-R 1 ; The resulting yellow solid powder UiO-66-R 1 Disperse 1.5 g in 100 ml of ethanol, add 0.75 g of p-aminobenzoic acid to obtain a mixed solution, reflux the mixed solution for 20 hou...
Embodiment 2
[0043] Take 0.5g of 2-aminoterephthalic acid and 0.5g of zirconium tetrachloride, dissolve them together in 150ml of N,N-dimethylformamide; then add 2.0ml of hydrochloric acid, stir well and then sonicate for 15 minutes , and then the mixed solution was transferred to a hydrothermal reaction kettle, heated to 120°C, and maintained at this temperature for 48 hours; naturally cooled to room temperature, filtered, washed with ethanol three times, and dried to obtain a white solid powder UiO-66- NH 2 ; The resulting white solid powder UiO-66-NH 2 Take 3.0 g and disperse it in 100 ml of ethanol, add 1.0 g of terephthalaldehyde to obtain a mixed solution, reflux the mixed solution for 10 hours, cool to room temperature, filter, wash with ethanol three times, and dry to obtain a yellow solid powder UiO -66-R 1 ; The resulting yellow solid powder UiO-66-R 1 Disperse 1.0 g in 100 ml of ethanol, add 0.3 g of p-aminobenzoic acid to obtain a mixed solution, reflux the mixed solution fo...
Embodiment 3
[0045] Take 2.0g of 2-aminoterephthalic acid and 2.0g of zirconium tetrachloride, dissolve them together in 150ml of N,N-dimethylformamide; then add 10.0ml of hydrochloric acid, stir well and then sonicate for 15 minutes , then transfer the mixed solution into a hydrothermal reaction kettle, heat to 120°C, and keep this temperature for 48 hours; after cooling to room temperature, filter, wash with ethanol three times and dry to obtain white solid powder UiO-66- NH 2 ; The resulting white solid powder UiO-66-NH 2 Take 1.0 g and disperse it in 100 ml of ethanol solution; add 0.3 g of terephthalaldehyde to obtain a mixed solution, and reflux the mixed solution for 10 hours; filter after cooling to room temperature, wash with ethanol three times and then dry to obtain a yellow solid powder UiO -66-R 1 ; The resulting yellow solid powder UiO-66-R 1 Disperse 3.0 g in 100 ml of ethanol, add 1.5 g of p-aminobenzoic acid to obtain a mixed solution, reflux the mixed solution for 20 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com