Synthesis method of 3,3-difluoro-4-pyrroline-2-one compound
A technology of ketone compound and synthesis method, which is applied in the field of chemical organic synthesis and achieves the effects of high yield, wide substrate range and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] First, put a stirring bar in a 35 mL sealed tube, and add 79.2 mg (0.3 mmol) bromodifluoroacetyl-p-toluidine, 3.0 ml acetonitrile, and 49 μL phenylacetylene (0.45 mmol) in sequence and mix well. Second, 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 82.9 mg K 2 CO 3 (0.6 mmol), sealed the mouth of the tube with a cock, heated to 110°C and stirred for 2 hours. After the reaction, the system was cooled to room temperature, 3 ml of distilled water was added to the reaction mixture, extracted with ethyl acetate (5 ml×3), the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and the residue was passed through a silica gel column. Chromatography (V 石油醚 :V 乙酸乙酯 =50:1) 78.7 mg of white solid product 3a was isolated with a yield of 92%. The reaction is shown in the following formula:
[0021]
[0022] Spectral analysis data
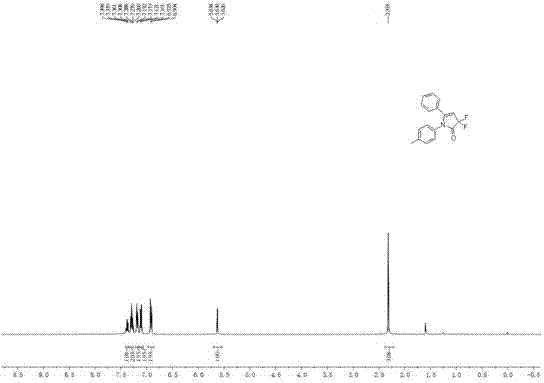
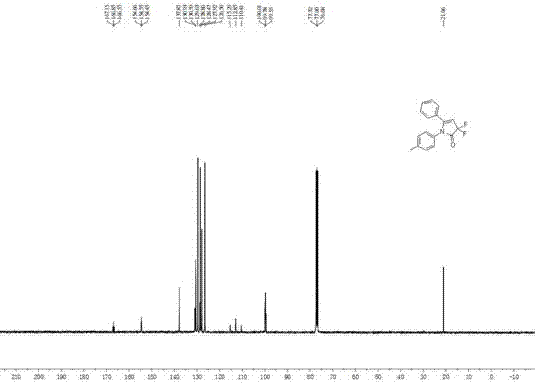
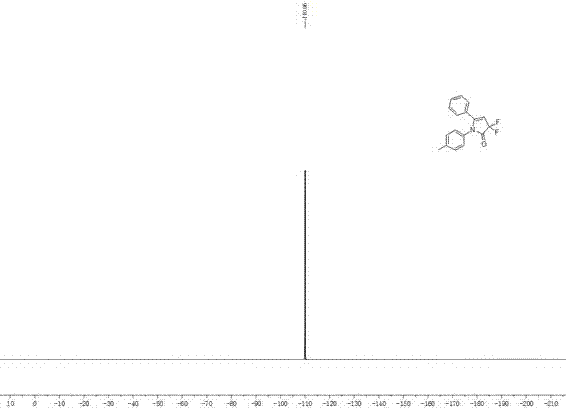
[0023] 1 H NMR (400 MHz, CDCl 3 ): δ = 2.32 (s, 3H), 5.63 (t, J ...
Embodiment 2
[0025]First, put a stirring bar in a 35 mL sealed tube, and add 79.2 mg (0.3 mmol) bromodifluoroacetyl-p-toluidine, 3.0 ml acetonitrile, and 52 μL p-fluorophenylacetylene (0.45 mmol) in sequence and mix well. Second, 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 82.9 mg K 2 CO 3 (0.6 mmol), seal the nozzle tightly with a cock, heat to 110°C and stir for 2 hours. After the reaction, the system was cooled to room temperature, 3 ml of distilled water was added to the reaction mixture, extracted with ethyl acetate (5 ml×3), the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and the residue was passed through a silica gel column. Chromatography (V 石油醚 :V 乙酸乙酯 =50:1) to isolate 82.9 mg of white solid product 3b with a yield of 91%. The reaction is shown in the following formula:
[0026]
[0027] Spectral analysis data
[0028] 1 H NMR (400 MHz, CDCl 3 ): δ = 2.33 (s, 3H), 5.61 (d, J = 1....
Embodiment 3
[0030] First, put a stirring bar in a 35 mL sealed tube, and add 79.2 mg (0.3 mmol) bromodifluoroacetyl-p-toluidine, 3.0 ml acetonitrile, and 81.5 mg p-bromophenylacetylene (0.45 mmol) in sequence and mix well. Second, 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 82.9 mg K 2 CO 3 (0.6 mmol), seal the nozzle tightly with a cock, heat to 110°C and stir for 2 hours. After the reaction, the system was cooled to room temperature, 3 ml of distilled water was added to the reaction mixture, extracted with ethyl acetate (5 ml×3), the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and the residue was passed through a silica gel column. Chromatography (V 石油醚 :V 乙酸乙酯 =50:1) 89.6 mg of white solid product 3c was isolated with a yield of 82%. The reaction is shown in the following formula:
[0031]
[0032] Spectral analysis data
[0033] 1 H NMR (400 MHz, CDCl 3 ): δ = 2.33 (s, 3H), 5.64 (s, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com