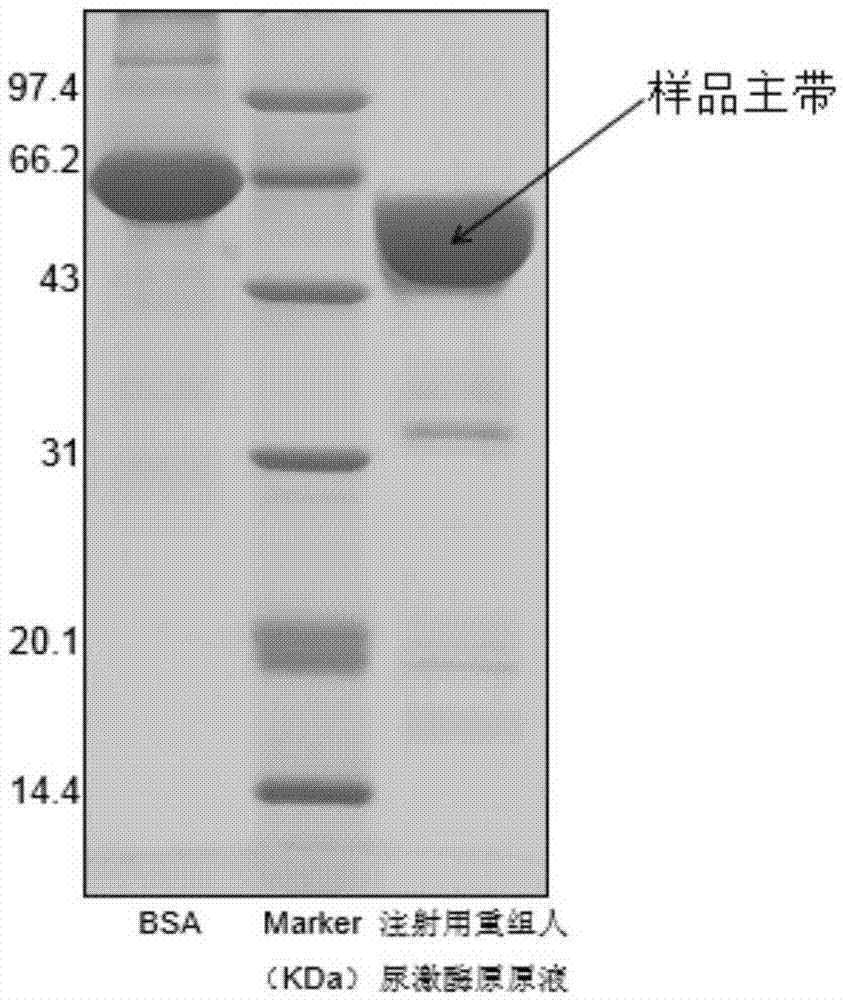
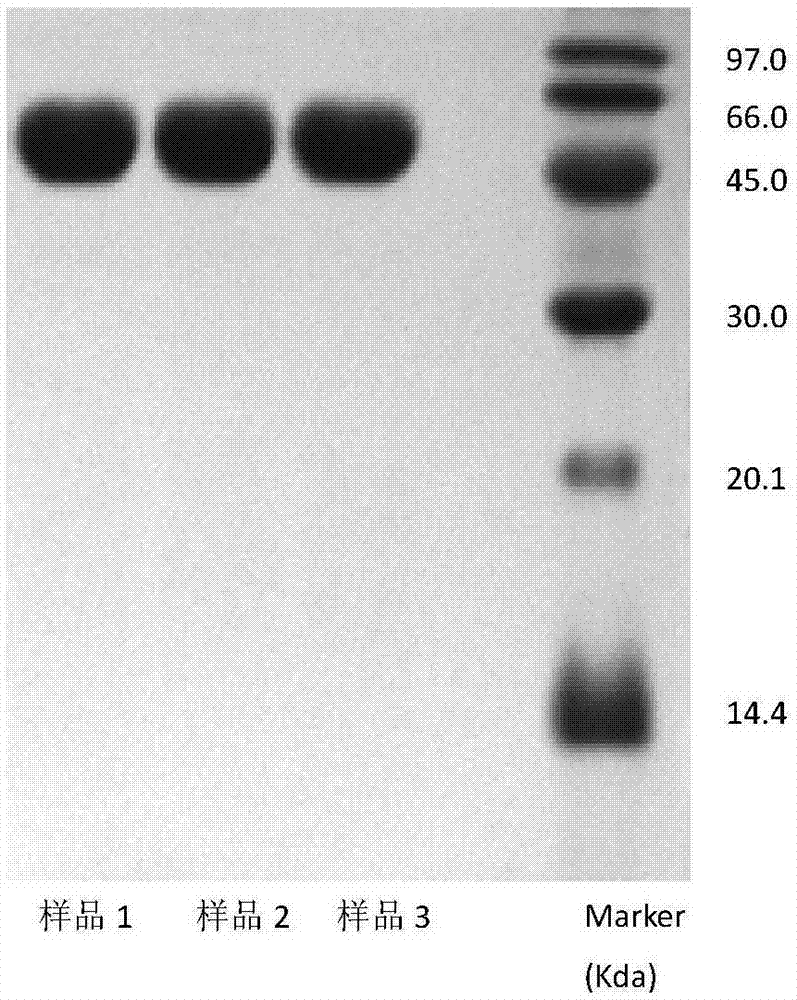
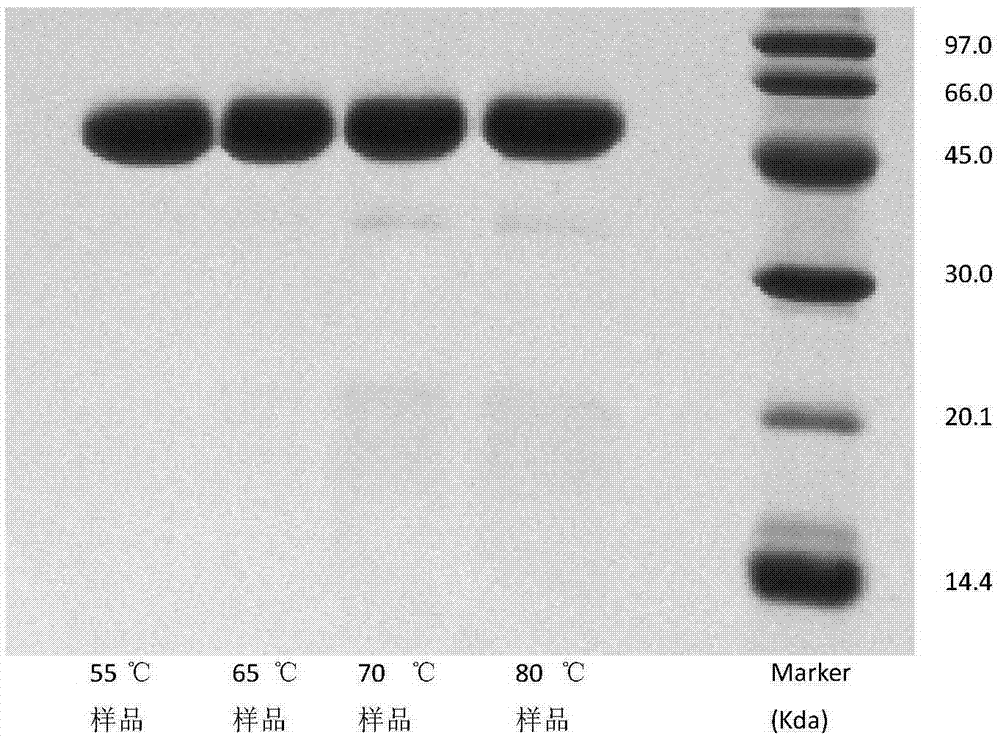
Method for electrophoresis determination of purity of recombinant human prourokinase for injection
A technology for prourokinase and injection, which is applied in the field of medicine and can solve problems such as unsatisfactory separation of test products, discomfort, and protein destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Testing of real samples:
[0084] 1. Operating procedures
[0085] 1.1 Reagents and solutions required for inspection
[0086] 1.1.1 30% gel stock solution: pure for electrophoresis, store in the dark at 2-8°C.
[0087] 1.1.2 1.5mol / L Tris-HCl buffer solution (pH8.8): Weigh 18.2g of trishydroxyaminomethane (Tris, pure by electrophoresis), add an appropriate amount of MilliQ water for injection to dissolve, adjust the pH to 8.8 with dilute hydrochloric acid, and constant volume To 100ml, store at 2-8°C.
[0088] 1.1.3 1.0mol / L Tris-HCl buffer (pH6.8): Weigh 12.1g of trishydroxyaminomethane (Tris, pure by electrophoresis), add an appropriate amount of MilliQ water for injection to dissolve, adjust the pH to 6.8 with dilute hydrochloric acid, and constant volume To 100ml, store at 2-8°C.
[0089] 1.1.4 10% Sodium Dodecyl Sulfate (SDS): Weigh 10 g of Sodium Dodecyl Sulfate (SDS, pure by electrophoresis), add 100 ml of MilliQ water for injection to dissolve, and store at...
Embodiment 2
[0170] The non-reducing sample buffer, electrophoresis buffer, 15% separating gel solution 5, and % stacking gel solution described in Example 1 are replaced as follows:
[0171] Non-reducing sample buffer refers to weighing 0.2g of trishydroxyaminomethane, 0.5g of sodium lauryl sulfate, and 0.001g of bromophenol blue, measuring 2ml of glycerol, and 0.05ml of hydrochloric acid, adding MilliQ water for injection to dissolve and constant volume. Can.
[0172] Electrophoresis buffer: Weigh 10g of trishydroxyaminomethane, 65g of glycine, 4g of sodium lauryl sulfate, add MilliQ water for injection to dissolve, adjust the pH to 7.5-9.0 with hydrochloric acid, and continue to dilute with milliQ water to obtain the product.
[0173]15% separating gel solution includes MilliQ water for injection 1ml, 30% gel stock solution 1.5ml, Tris-HCl buffer (PH8.8, 1.5M) 1ml, 10% SDS 0.02ml, 10% AP 0.01ml, TEMED 0.001ml ;
[0174] The 5% concentrated gel solution includes MilliQ water for inject...
Embodiment 3
[0176] The non-reducing sample buffer, electrophoresis buffer, 15% separating gel solution 5, and % stacking gel solution described in Example 1 are replaced as follows:
[0177] Non-reducing sample buffer refers to weighing 0.5g of trishydroxyaminomethane, 1g of sodium lauryl sulfate, and 0.003g of bromophenol blue, measuring 6ml of glycerol, and 1ml of hydrochloric acid, adding MilliQ water for injection to dissolve and constant volume.
[0178] Electrophoresis buffer: Weigh 20g of trishydroxyaminomethane, 85g of glycine, 8g of sodium lauryl sulfate, add MilliQ water for injection to dissolve, adjust the pH to 7.5-9.0 with hydrochloric acid, and continue to dilute with milliQ water to obtain the product.
[0179] Described 15% separation gel solution comprises MilliQ water for injection 2.5ml, 30% gel stock solution 3.5ml, Tris-HCl buffer (PH8.8, 1.5M) 2.5ml, 10%SDS0.02-0.1 part, 10 %AP 0.01-0.05 part, TEMED0.005ml; The 5% concentrated gel solution includes MilliQ water for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com