Lithium-sulfur battery
A lithium-sulfur battery and electrolyte technology, applied in the application field of lithium-sulfur batteries, can solve the problems of reduced conductivity of the electrolyte, difficult to withstand the battery, large volume change, etc., to solve the problem of diffusivity, increase instability, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
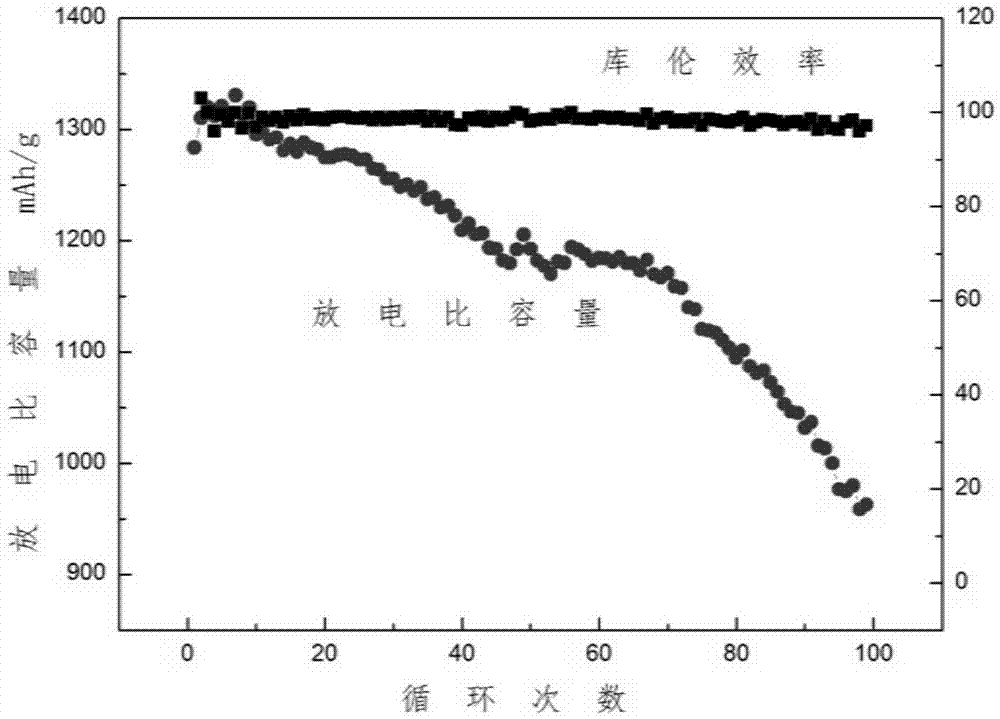
[0035] Add 0.25g of 1,1,2,2-tetrafluoroethanesulfonic acid 1-ethyl-3-methylimidazole into 10g of sulfolane solvent, and then add lithium bistrifluoromethanesulfonimide to make the concentration of lithium ions to 1M, vigorously stirred to obtain a clear electrolyte. The electrolyte solution obtained above was used to impregnate the positive electrode, and the positive electrode was a carbon-sulfur composite (58% sulfur filling, PVDF binder). The battery was charged and discharged at a rate of 0.1 for 100 cycles, the average Coulombic efficiency was 99.9%, and the capacity retention rate was 89% ( figure 1 );
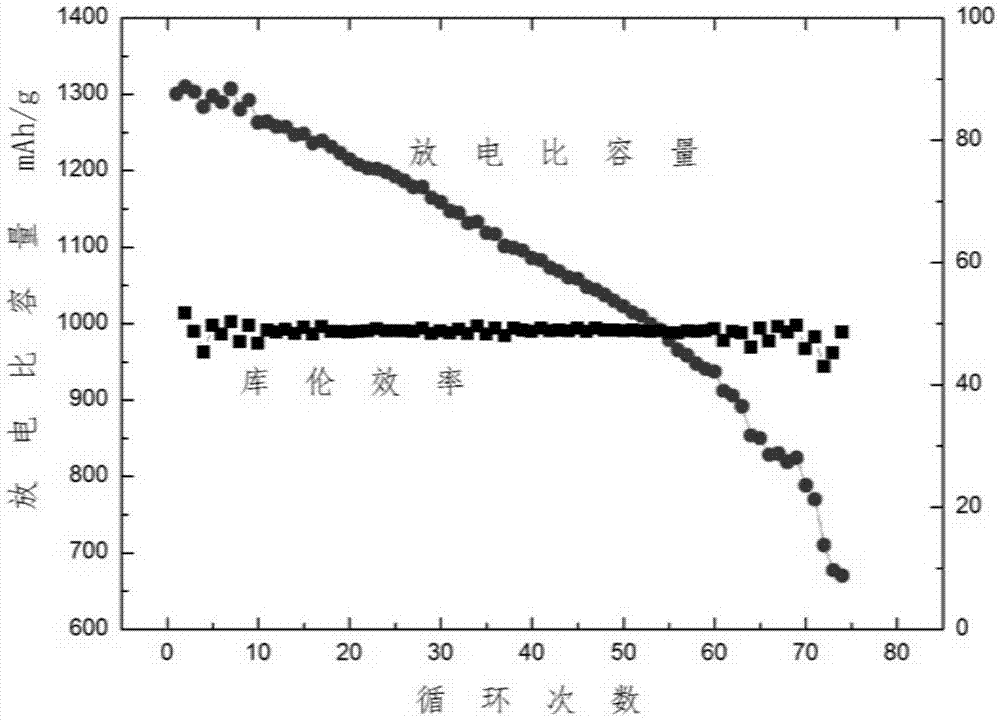
[0036] And when adopting the electrolytic solution without additive, other conditions are constant, the average coulombic efficiency of battery only has 48%, and capacity retention rate is 40% (( figure 2 ).
Embodiment 2
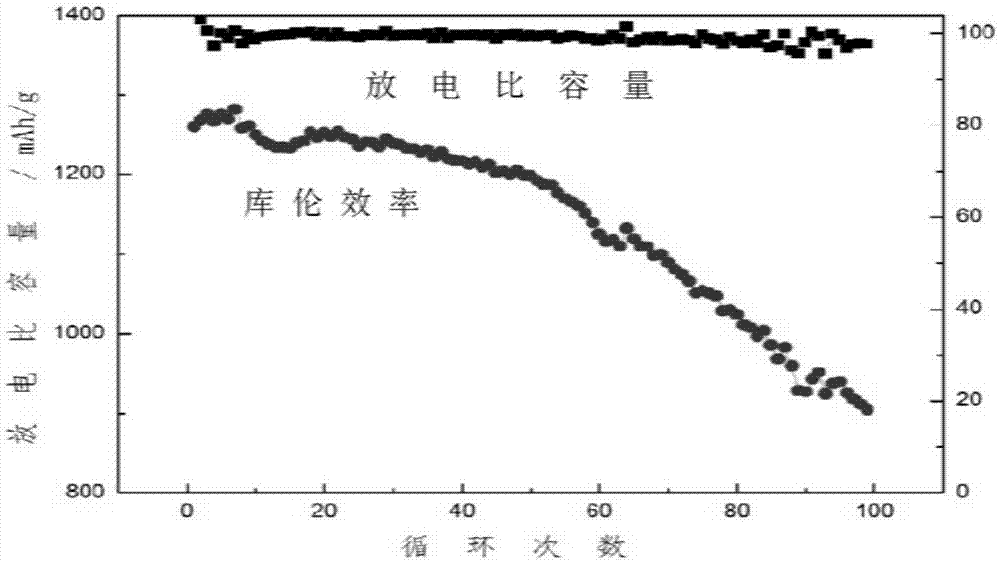
[0038] 5 g of triethylpyridine bromide was added to 10 g of sulfolane solvent, and then lithium bistrifluoromethanesulfonimide was added to make the concentration of lithium ions 1 M, and the clarified electrolyte was obtained by vigorous stirring. A lithium-sulfur button battery was assembled using the electrolyte solution obtained above, and its positive electrode was a carbon-sulfur composite (58% sulfur filling, PVDF binder). The battery was charged and discharged at a rate of 0.1 for 100 cycles, the average Coulombic efficiency was 99.9%, and the capacity retention rate was 90% ( image 3 ).
Embodiment 3
[0040] Add 0.504 g of tributyldodecylphosphonium bromide salt to 10 g of sulfolane solvent, then add lithium bistrifluoromethanesulfonyl imide to make the concentration of lithium ions 1M, and stir vigorously to obtain a clear electrolyte. A lithium-sulfur button battery was assembled using the electrolyte solution obtained above, and its positive electrode was a carbon-sulfur composite (58% sulfur filling, PVDF binder). The battery was charged and discharged at a rate of 0.1 for 37 cycles, the average Coulombic efficiency was 100%, and the capacity retention rate was 80% ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com