Preparation method for amorphous carbon positive electrode of lithium-air battery and lithium-air battery
A lithium-air battery, non-carbon technology, applied in the field of electrochemical energy, can solve the problems of reducing Coulombic efficiency, affecting battery performance, and easy decomposition of carbon materials, so as to reduce the overpotential of charge and discharge, improve the sharp drop of discharge voltage, and easily decompose Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The embodiment of the present invention provides a method for preparing a non-carbon positive electrode of a lithium-air battery, comprising the following steps:
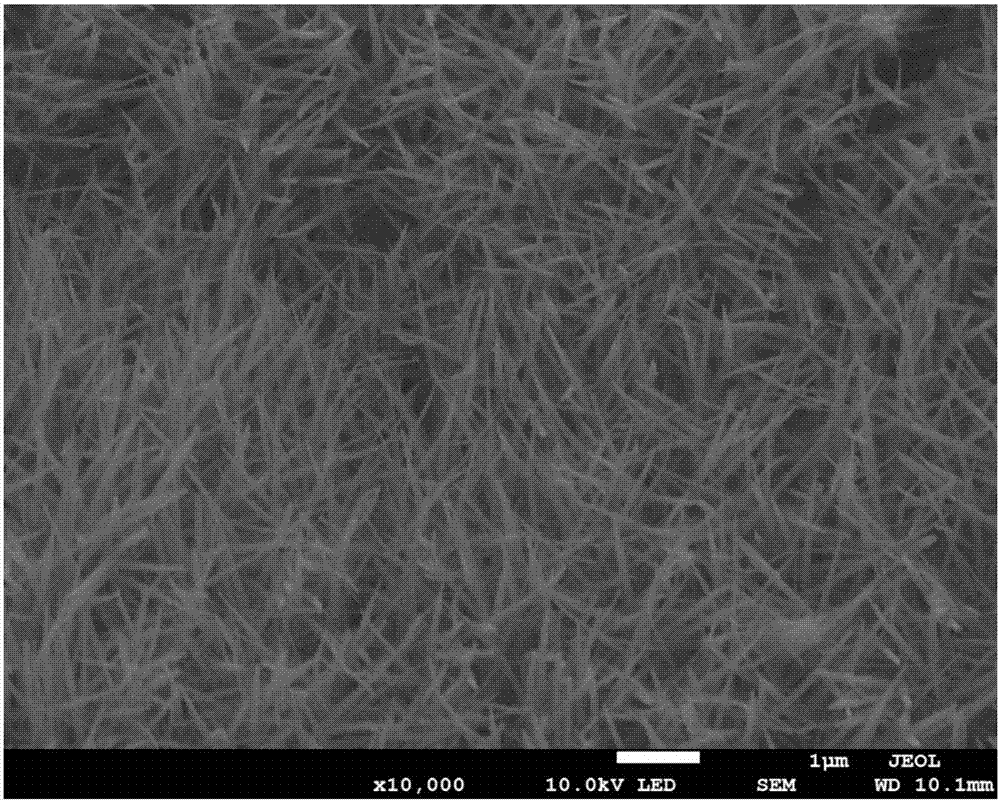
[0037] a. Growth of Co on Ni foam substrate by hydrothermal reaction 3 o 4 Precursor;
[0038] b. Calcining in air, the Co 3 o 4 The precursor is transformed into Co 3 o 4 , forming Co 3 o 4 @Ni non-carbon positive electrode (loaded Co 3 o 4 foam nickel electrode);
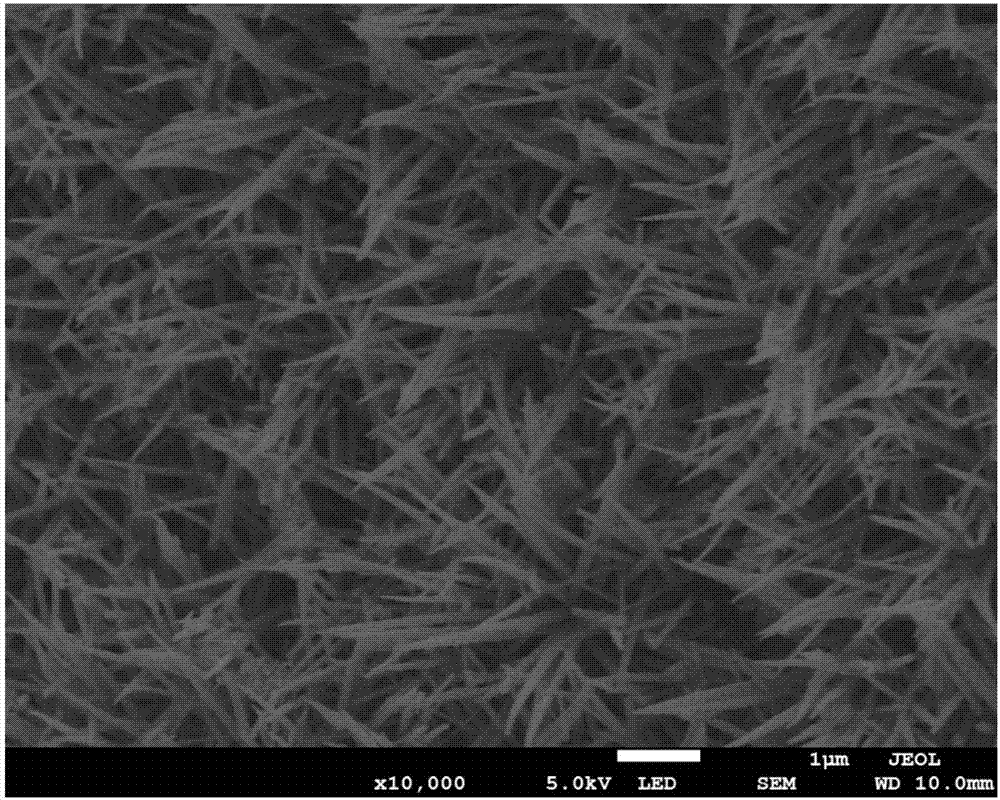
[0039] c. First load Ni with Co 3 o 4 Soak in RuCl 3 solution, followed by high-temperature treatment under the protection of argon to obtain RuO 2 / Co 3 o 4 @Ni non-carbon cathode (RuO 2 Modified Co 3 o 4 @Ni electrode).
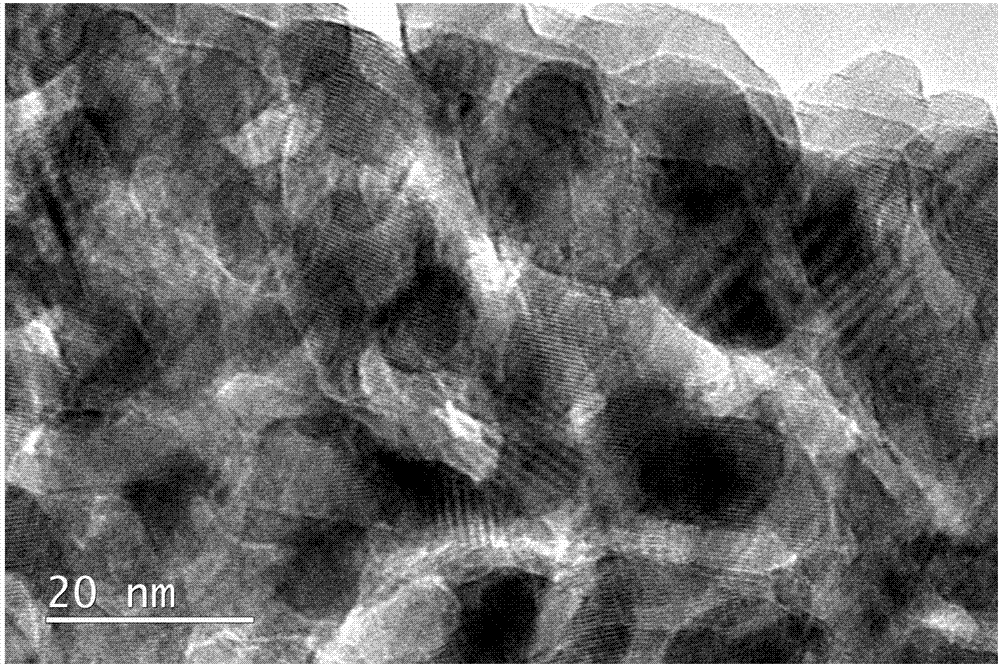
[0040] The preparation method of the lithium-air battery non-carbon positive electrode provided by the embodiment of the present invention uses a hydrothermal process to make nanowire-like Co 3 o 4 The precursor is grown on the foamed nickel substrate, and the precursor is converted into the metal oxide Co...
Embodiment 1
[0066] The pretreatment process of foamed nickel is: cut the untreated foamed nickel into 3.2*5cm 2 The rectangular slices were placed in an acetone solution and ultrasonically removed for 5 minutes to remove surface oil stains. After multiple rinses with deionized water, they were ultrasonically cleaned with 3M HCl for 15 minutes to remove surface oxides. Then rinse with absolute ethanol and deionized water for 3-5 times in turn, and dry in an oven for use.
[0067] The preparation process of the positive electrode is: sequentially weigh 0.8731g of Co(NO 3 ) 2 ·6H 2 O (3mmol) and 0.72g of urea (12mmol) were placed in a clean 100mL beaker, poured into 40mL of deionized water, and stirred magnetically until the raw materials were completely dissolved and the solution was pink. Transfer the solution to a 50mL reaction kettle with a polytetrafluoroethylene liner, place the dry nickel foam rectangle piece along the inner wall of the liner, and lock the reaction kettle. Place t...
Embodiment 2
[0070] Foam nickel pretreatment process is identical with embodiment 1
[0071] The manufacturing process of the positive electrode:
[0072] The first step, a hydrothermal process, allowed the growth of Co on the foamed nickel substrate 3 o 4 Precursor:
[0073] Sequentially weigh 0.8731g of Co(NO 3 ) 2 ·6H 2 O (3mmol) and 0.72g of urea (12mmol) were placed in a clean 100mL beaker, poured into 40mL of deionized water, and stirred magnetically until the raw materials were completely dissolved and the solution was pink. Transfer the solution to a 50mL reaction kettle with a polytetrafluoroethylene liner, place the dry nickel foam rectangle piece along the inner wall of the liner, and lock the reaction kettle. Place the reaction kettle in an oven at 100-120°C for 6 hours. After the hydrothermal treatment, the reactor was naturally cooled, and the nickel foam loaded with the precursor was taken out, rinsed with absolute ethanol and deionized water for 3-5 times, and dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com