Near-infrared iodo-bodipy photosensitizer and preparation method thereof
A fluoroboron dipyrrole photosensitizer and a technology for fluoroboron dipyrrole, which are applied in the field of near-infrared iodo fluoroboron dipyrrole photosensitizers and the preparation thereof, can solve the problems of many synthesis steps, poor solubility, limited application and the like, and achieve The effect of low cell damage, easy modification and high molar absorptivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
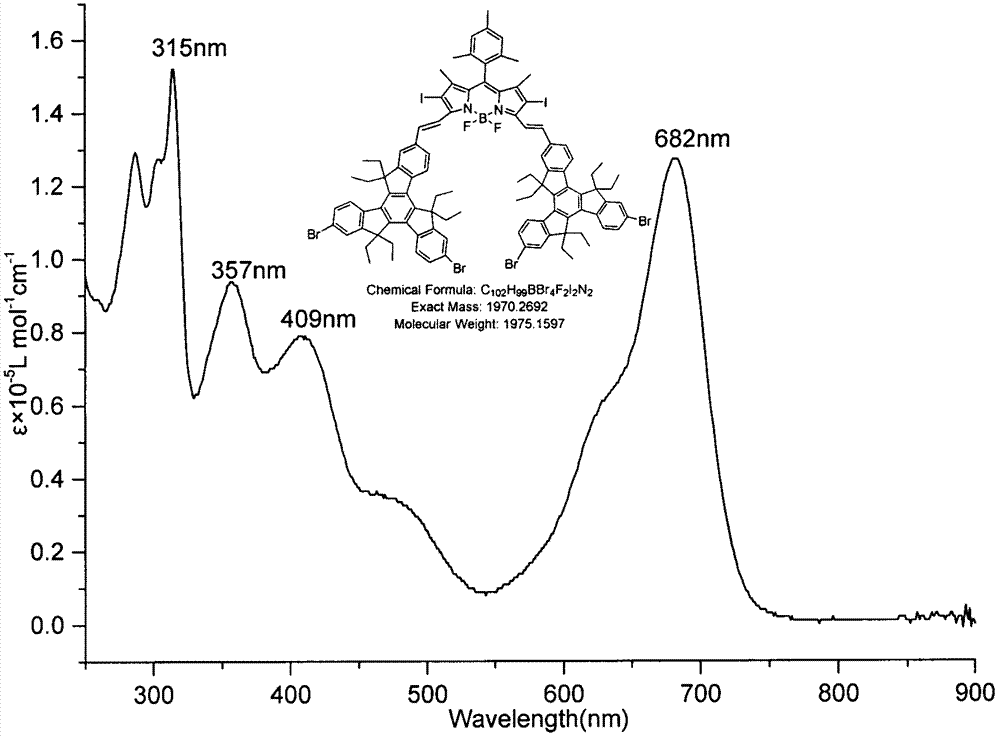
[0028] Equipped with a water separator in the round bottom flask, 2,6-diiodo BODIPY (123.6mg, 0.20mmol), 2-formyl-6,10-dibromotripolyindene (0.138g, 0.2mmol) and p- Toluenesulfonic acid (40mg) was dissolved in 25mL toluene and 2mL piperidine, the mixture was heated to reflux at 140°C, and the solvent was collected until evaporated to dryness. The reactant was concentrated and subjected to silica gel column chromatography, the eluent was (petroleum ether: CH 2 Cl 2 =1:1), a green solid A (113.7 mg, 28.79%) was obtained. Esi-MS: calcd for C 102 h 99 BBr 4 f 2 I 2 N 2 1975.1597, found: 1974.27598 (M - ); 1 H NMR: (600MHz, CDCl 3 )δ8.36(t, J=7.80Hz, 3H), 8.33(s, 1H), 8.19(t, J=9.00Hz, 4H), 7.92(s, 1H), 7.89(s, 1H), 7.79( d, J=7.20Hz, 2H), 7.69(s, 2H), 7.59(d, J=1.80Hz, 2H), 7.53-7.50(m, 4H), 7.37-7.35(m, 2H), 703(s , 2H), 3.02-2.92(m, 12H), 2.40(s, 3H), 2.29-2.26(m, 4H), 2.15-2.06(m, 14H), 1.53(s, 6H), 0.30-0.21(m , 36H); UV-vis: 315nm, 357nm, 409nm, 682nm ( figure 1...
Embodiment 2
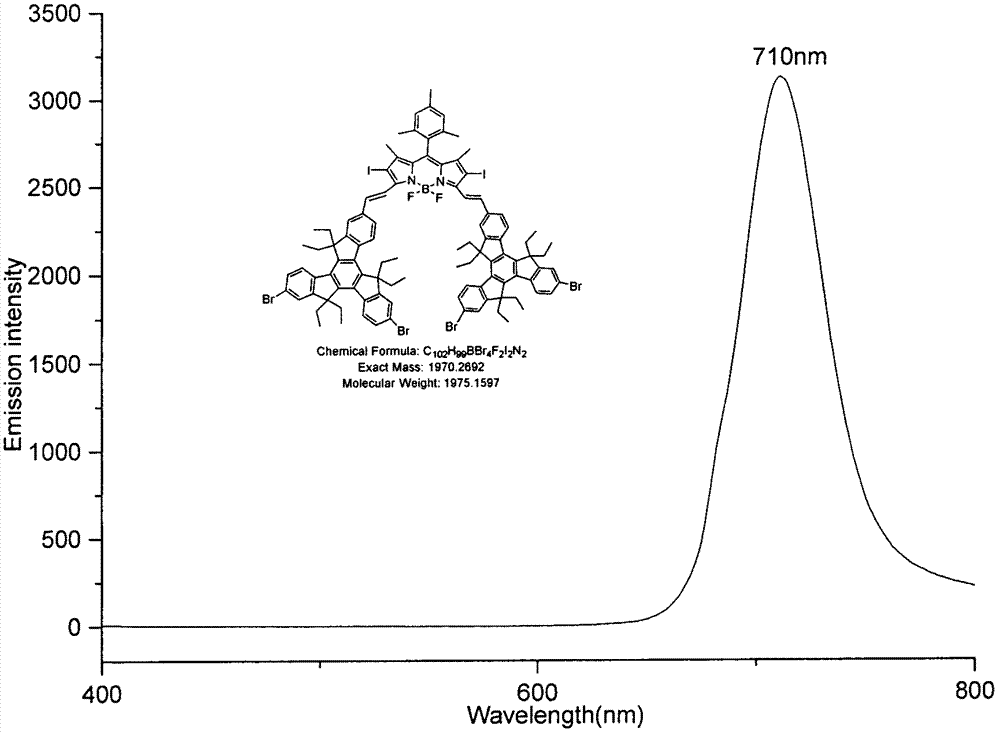
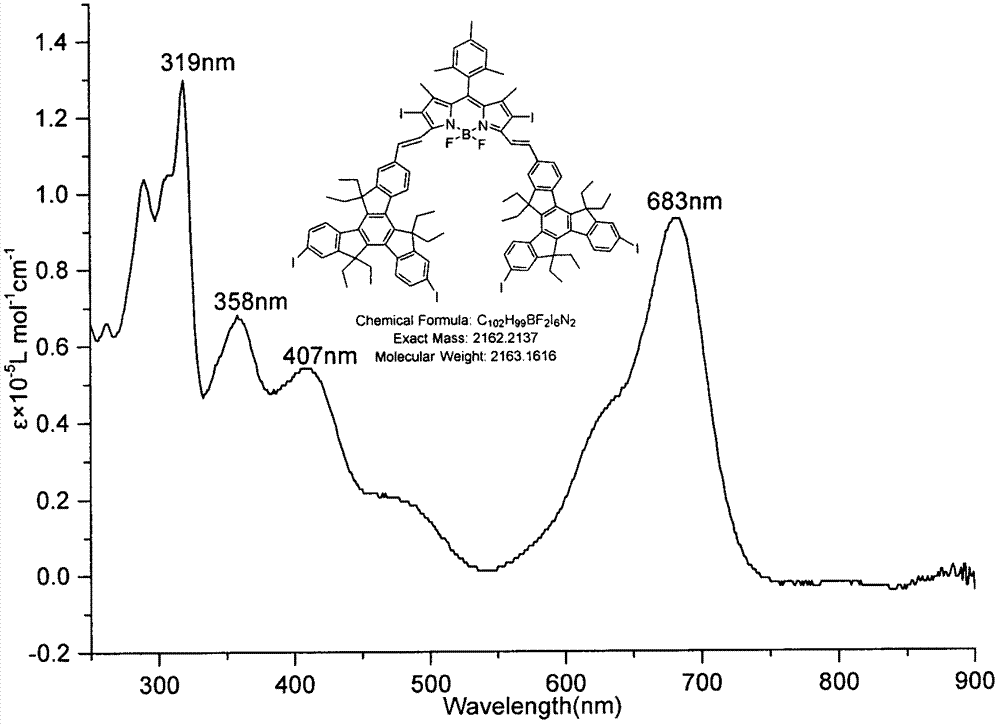
[0030] Equipped with a water trap in the round bottom flask, 2,6-diiodo BODIPY (185.4mg, 0.30mmol), 2-formyl-6,10-diiodotripolyindene (237.7mg, 0.30mmol) and p Toluenesulfonic acid (50 mg) was dissolved in 25 mL of toluene and 2 mL of piperidine, the mixture was heated to reflux at 140°C, and the solvent was collected until evaporated to dryness. The reactant was concentrated and subjected to silica gel column chromatography, and the eluent was (petroleum ether / CH 2 Cl 2 =7:3), to obtain green solid B (89.7mg, 13.82%). Esi-MS: calcd for C 102 h 99 BF 2 I 6 N 2 2163.16, found: 2163.15 (M + ); 1 H NMR: (600MHz, CDCl 3 )δ8.36(t, J=9.00Hz, 4H), 8.07(t, J=9.00Hz, 4H), 7.91(s, 1H), 7.88(s, 1H), 7.80-7.77(m, 4H), 7.73-7.69(m, 6H), 7.56(d, J=8.40Hz, 2H), 7.03(s, 2H), 3.01-2.90(m, 12H), 2.40(s, 3H), 2.28-2.25(m, 4H), 2.15-2.05(m, 14H), 1.53(s, 6H), 0.29-0.21(m, 36H); UV-vis: 319nm, 358nm, 407nm, 683nm( image 3 ); Emission Wavelength: 710nm ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com