Synthesis method of gastrodin
A synthesis method and technology of gastrodin, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., to achieve the effects of low cost, stable quality, safe and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
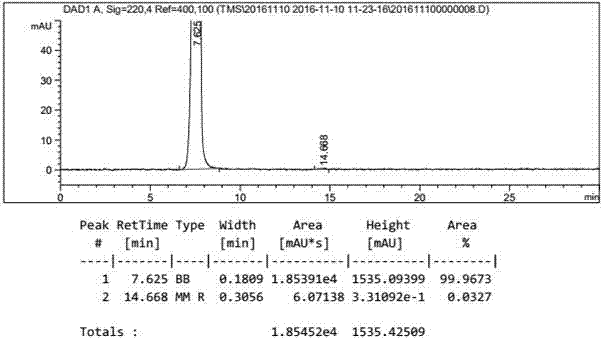
[0047] Add 300g of ethyl acetate to the reactor, then add 50g of potassium triacetoxyborohydride, then add 100g of 4-formylphenyl-2,3,4,6-tetra-0-acetyl-β-D-pyran Glucoside, the temperature is controlled at 20 to 30°C and the reaction is kept warm. Sampling is used to monitor the reaction by TLC. After 1 hour of reaction, the reaction is complete. The reaction solution is added to 300ml of water to quench, and then the upper organic phase is separated, and the organic phase is concentrated to a solid under vacuum. , then add 300g methanol and 22g diethylamine for reflux reaction for 2 hours, take a sample to monitor the reaction with TLC, add 10g activated carbon to decolorize for 10 minutes after the reaction is complete, filter, concentrate the filtrate to 1 / 3 of the original volume, and then add to the reaction solution Add 300g of acetone, stir and crystallize for 1 hour, filter to obtain 70g of gastrodin warm product, and dry at 70°C to obtain 55g of gastrodin dry product....
Embodiment 2
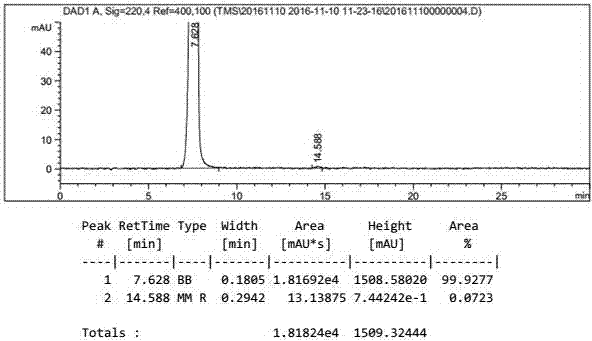
[0049] Add 300g of ethyl acetate to the reactor, then add 50g of sodium triacetoxyborohydride, then add 100g of 4-formylphenyl-2,3,4,6-tetra-0-acetyl-β-D-pyran The temperature of glucoside is controlled at 20 to 30°C for heat preservation reaction, and the reaction is monitored by TLC for sampling. After 2 hours of reaction, the reaction is complete. The reaction solution is added to 300ml of water to quench, and then the upper organic phase is separated, and the organic phase is concentrated to a solid under vacuum. Then add 300g methanol and 22g diethylamine for reflux reaction for 2 hours, take a sample to monitor the reaction with TLC, add 10g activated carbon to decolorize for 10 minutes after the reaction is complete, filter, concentrate the filtrate to 1 / 3 of the original volume, and then pour it into the reaction solution Add 300g of acetone, stir and crystallize for 1 hour, filter to obtain 65g of gastrodin warm product, and dry at 70°C to obtain 52g of gastrodin dry p...
Embodiment 3
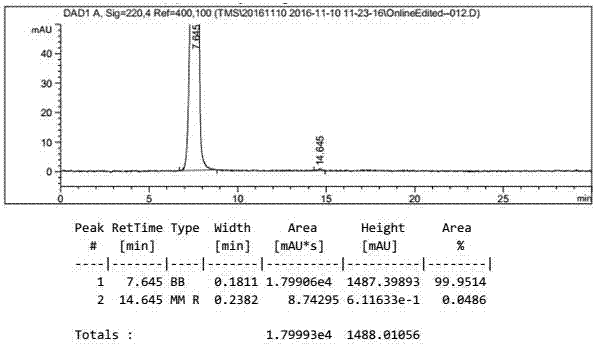
[0051] Add 200g of ethyl acetate to the reactor, then add 50g of potassium triacetoxyborohydride, then add 100g of 4-formylphenyl-2,3,4,6-tetra-0-acetyl-β-D-pyran The temperature of glucoside is controlled at 20 to 30°C for heat preservation reaction, and the reaction is monitored by TLC for sampling. After 1.5 hours of reaction, the reaction is complete. The reaction solution is added to 300ml of water to quench, and then the upper organic phase is separated, and the organic phase is concentrated to a solid under vacuum. Then add 300g methanol and 22g diethylamine for reflux reaction for 2 hours, take a sample to monitor the reaction with TLC, add 10g activated carbon to decolorize for 10 minutes after the reaction is complete, filter, concentrate the filtrate to 1 / 3 of the original volume, and then pour it into the reaction solution Add 300g of acetone, stir and crystallize for 1 hour, filter to obtain 68g of gastrodin warm product, and dry at 70°C to obtain 53g of gastrodin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com