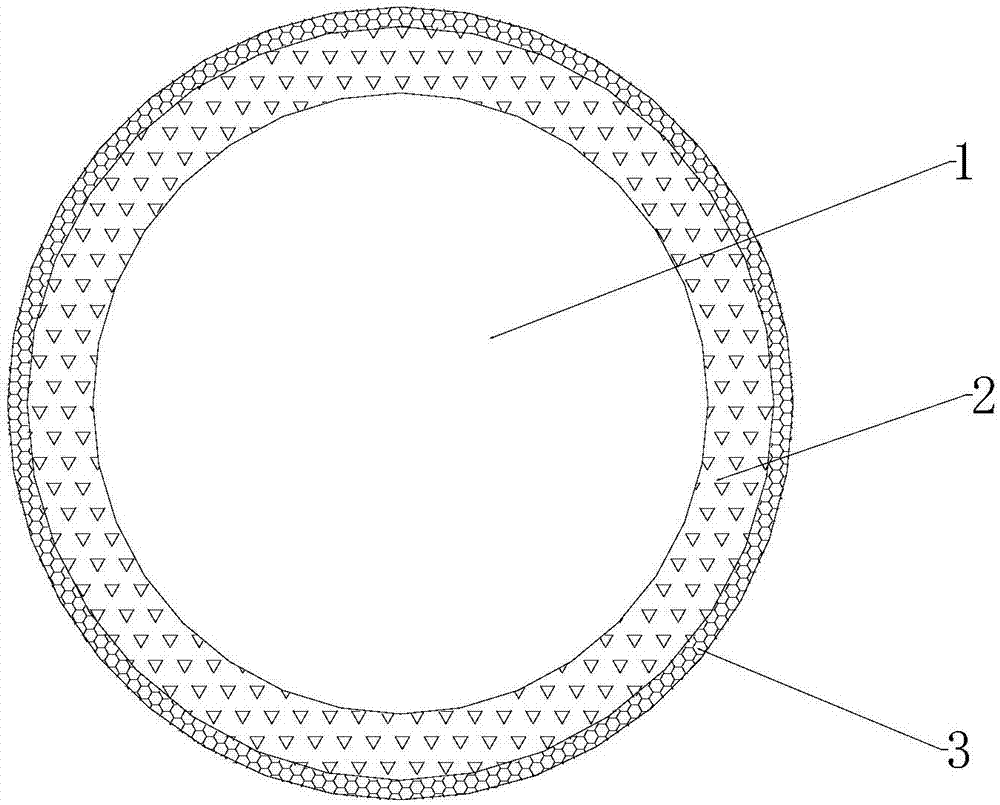
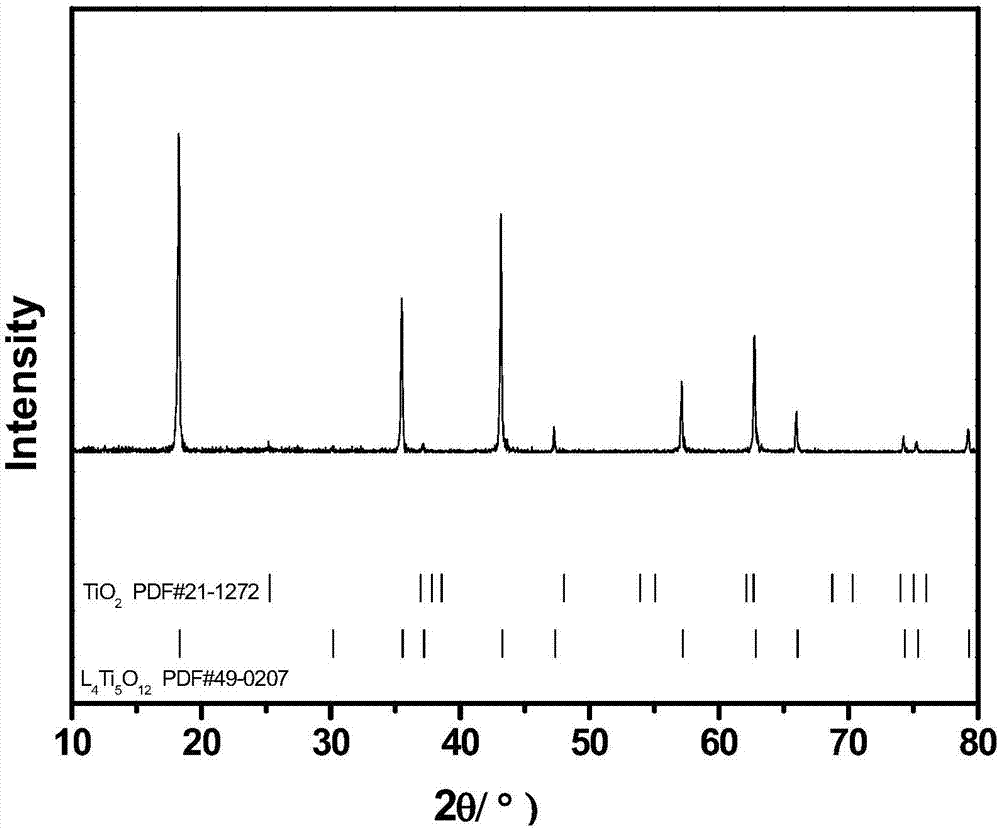
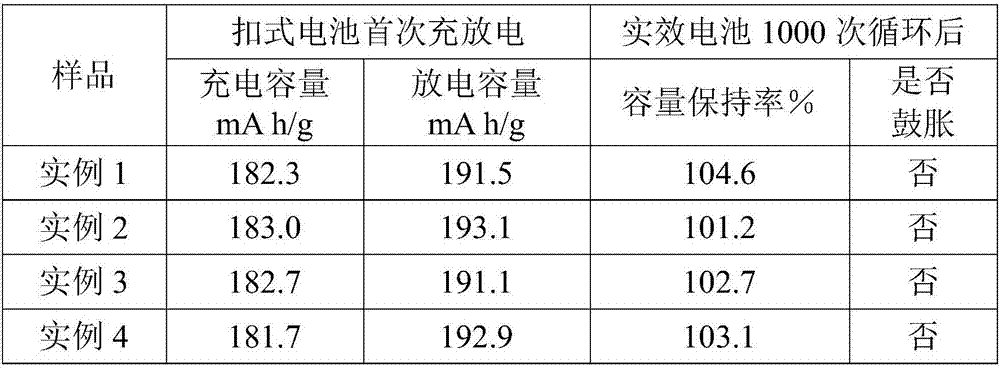
Lithium titanate-titanium dioxide composite material formed in situ and preparation method thereof
A titanium dioxide, in-situ generation technology, applied in the direction of secondary batteries, electrochemical generators, electrical components, etc., can solve the problems of poor mixing uniformity of lithium titanate, affecting product performance, affecting applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing lithium titanate-titanium dioxide composite material in situ, comprising the steps of:
[0028] 1) adding titanium dioxide to 10 mol / l sodium hydroxide solution, in a hydrothermal reaction kettle, keeping the temperature at 180° C. for 48 hours to obtain material I;
[0029] Wherein the mol ratio of titanium dioxide and sodium hydroxide is 1:6;
[0030] 2) Add material I to 0.4mol / L [H + ] in the hydrochloric acid solution, wherein material I / acid solution=1g / 200ml, the reaction time is 48h, obtain ion exchange solution I;
[0031] 3) Put the ion exchange solution I into a high-speed centrifuge for washing and separation, collect the solids, and obtain the material II;
[0032] 4) Add the material II to a 1mol / l lithium hydroxide solution, mix well, add it to a hydrothermal reactor, and keep it warm at 150°C for 24 hours to obtain the ion replacement solution II;
[0033] 5) Wash and separate the ion exchange solution II in a high-speed centrif...
Embodiment 2
[0040] A method for preparing lithium titanate-titanium dioxide composite material in situ, comprising the steps of:
[0041] 1) adding titanium dioxide to 10 mol / l sodium hydroxide solution, in a hydrothermal reaction kettle, keeping the temperature at 210° C. for 18 hours to obtain material I;
[0042] Wherein the mol ratio of titanium dioxide and sodium hydroxide is 1:12;
[0043] 2) Add material I to 0.8mol / L [H + ] in the hydrochloric acid solution, wherein material I / acid solution=1g / 100ml, the reaction time is 12h, obtain ion replacement solution I;
[0044] 3) Put the ion exchange solution I into a high-speed centrifuge for washing and separation, collect the solids, and obtain the material II;
[0045] 4) Add the material II to a 2mol / l lithium hydroxide solution, mix it evenly, add it into a hydrothermal reactor, and keep it at 200°C for 12 hours to obtain the ion replacement solution II;
[0046] 5) Wash and separate the ion exchange solution II in a high-speed c...
Embodiment 3
[0053]1) adding titanium dioxide to 10mol / l sodium hydroxide solution, in a hydrothermal reaction kettle, keeping the temperature at 200°C for 36h to obtain material I;
[0054] Wherein the mol ratio of titanium dioxide and sodium hydroxide is 1:18;
[0055] 2) Add material I to 0.8mol / L [H + ] in the sulfuric acid solution, wherein material I / acid solution=1g / 150ml, the reaction time is 36h, obtain ion exchange solution I;
[0056] 3) Put the ion exchange solution I into a high-speed centrifuge for washing and separation, collect the solids, and obtain the material II;
[0057] 4) Add the material II to a 2mol / l lithium hydroxide solution, mix it evenly, add it to a hydrothermal reactor, and keep it at 180°C for 18 hours to obtain the ion replacement solution II;
[0058] 5) Wash and separate the ion exchange solution II in a high-speed centrifuge, collect the solids, and obtain the material III;
[0059] 6) Roasting the material III once at 800° C. for 12 hours to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com