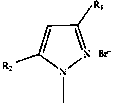
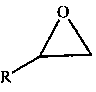
A kind of protonated pyrazole ionic liquid and the method of using it to catalyze the synthesis of cyclic carbonate
A technology of cyclic carbonates and ionic liquids, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve complex synthesis process, harsh reaction conditions, catalyst Expensive and other issues, to achieve the effect of simple synthesis process, high reactivity, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0016] Example 1-1: The protonated pyrazole ionic liquid is 1-methylpyrazole hydrobromide.
[0017] The preparation method of 1-methylpyrazole hydrobromide in the present embodiment is as follows:
[0018] Add 1.23g (15mmol) of 1-methylpyrazole and 3.102g (18mmol) of hydrobromic acid into a 100mL three-necked flask respectively, and stir for 15-30h; Yellow solid 1-methylpyrazole hydrobromide ionic liquid, yield 93%.
[0019] 1 H NMR (400MHz, DMSO-d 6 )δ: 7.78(d, J=2.2Hz, 1H), 7.55(d, J=2.0Hz, 1H), 6.29(t, J=2.2Hz, 1H), 5.30(s, 8H), 3.86(s, 3H).
[0020] 13 C NMR (101MHz, D 2 O)δ:136.16,134.44,107.76,37.72.MS(ESI):m / z:83[M-Br] + .
Embodiment 1-2
[0021] Example 1-2: The protonated pyrazole ionic liquid is 1,3-dimethylpyrazole hydrobromide.
[0022] In the present embodiment, the preparation method of 1,3-dimethylpyrazole hydrobromide is as follows:
[0023] Add 1.442g (15mmol) of 1,3-dimethylpyrazole and 3.102g (18mmol) of hydrobromic acid into a 100mL three-neck flask respectively, and stir for 15-30h; after the reaction, remove water, and wash the product with ethyl acetate. Drying gave white solid 1,3-dimethylpyrazole hydrobromide ionic liquid with a yield of 83%.
[0024] 1 H NMR (400MHz, DMSO-d 6 )δ: 7.83(d, 1H), 6.22(d, J=2.6Hz, 1H), 3.85(s, J=2.0Hz, 3H), 2.23(s, 3H).
[0025] 13 C NMR (101MHz, D 2 O)δ: 146.04, 136.48, 107.18, 37.31, 10.26. MS (ESI): m / z: 97 [M-Br] + .
Embodiment 1-3
[0026] Example 1-3: The protonated pyrazole ionic liquid is 1,5-dimethylpyrazole hydrobromide.
[0027] In the present embodiment, the preparation method of 1,5-dimethylpyrazole hydrobromide is as follows:
[0028] Add 1.442g (15mmol) of 1,5-dimethylpyrazole and 3.102g (18mmol) of hydrobromic acid into a 100mL three-neck flask respectively, and stir for 15-30h; after the reaction, remove water, and wash the product with ethyl acetate. Drying gave white solid 1,5-dimethylpyrazole hydrobromide ionic liquid with a yield of 70%.
[0029] 1 H NMR (400MHz, DMSO-d 6 )δ: 7.47(d,1H),6.14(d,J=1.9Hz,1H),3.75(d,J=2.1Hz,3H),2.27(d,J=2.6Hz,3H).
[0030] 13 C NMR (101MHz, D 2 O)δ: 146.08, 132.88, 107.70, 34.86, 10.19. MS (ESI): m / z: 97 [M-Br] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com