TPBG antibody, and preparation method, conjugate and application thereof
An antibody and sequence technology, applied in the field of TPBG antibody and its preparation, can solve the problems of non-cancerous cell damage and the limitation of killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] The preparation of embodiment 1TPBG antibody
[0199] The nucleotide sequence containing the amino acid sequence 32-355 (Ser32-Ser355) of the extracellular region encoding the human source TPBG protein (wherein the nucleotide sequence encoding the human source TPBG protein is Genebank ID: AAH37161.1 in Genebank) Cloning into pCpC vector (purchased from Invitrogen, V044-50) carrying human IgG Fc fragment (hFc) and plasmid preparation according to established standard molecular biology methods. For specific methods, see Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Second Edition (Plainview, New York: Cold Spring Harbor Laboratory Press). HEK293 cells (purchased from Invitrogen) were transiently transfected (polyetherimide PEI, purchased from Polysciences) and expanded at 37° C. using FreeStyle™ 293 (purchased from Invitrogen). After 4 days, the cell culture fluid was collected, and the cell components were removed by centr...
Embodiment 2
[0217] Example 2 Production and Purification of Lead Antibody
[0218] The concentration of antibodies produced by hybridoma cells is low, only about 1-10 μg / mL, and the concentration varies greatly. Moreover, various proteins produced by cell culture in the culture medium and fetal bovine serum components contained in the culture medium have varying degrees of interference with many biological activity analysis methods, so small-scale (1-5 mg) antibody production and purification are required.
[0219] The hybridoma cells obtained in Example 1 were inoculated into T-75 cell culture flasks and acclimatized and passaged for 3 generations with a production medium (Hybridomaserum free medium, purchased from Invitrogen). When the growth state is good, inoculate the cell culture spinner bottle. Add 200mL of production medium to each 2-liter culture spinner bottle, and inoculate the cell density at 1.0╳10 5 / mL. Close the bottle cap tightly, and place the spinner bottle on a spin...
Embodiment 3
[0225] The assay of embodiment 3 leading antibody
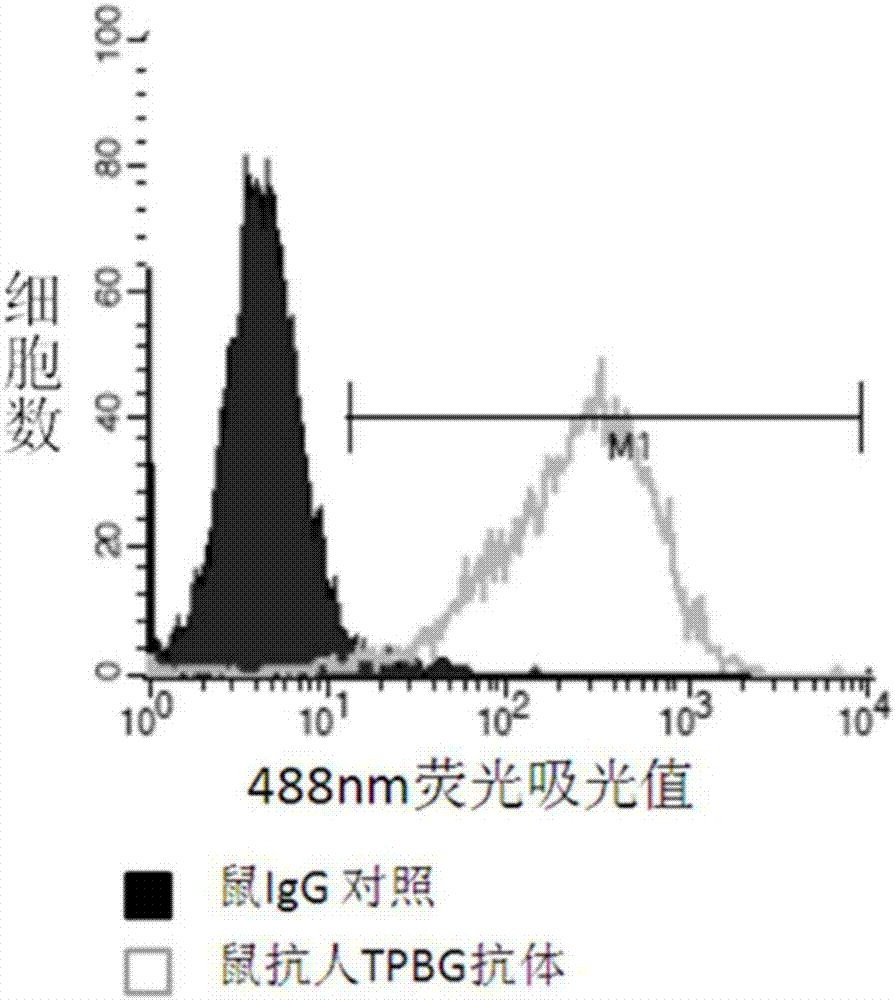
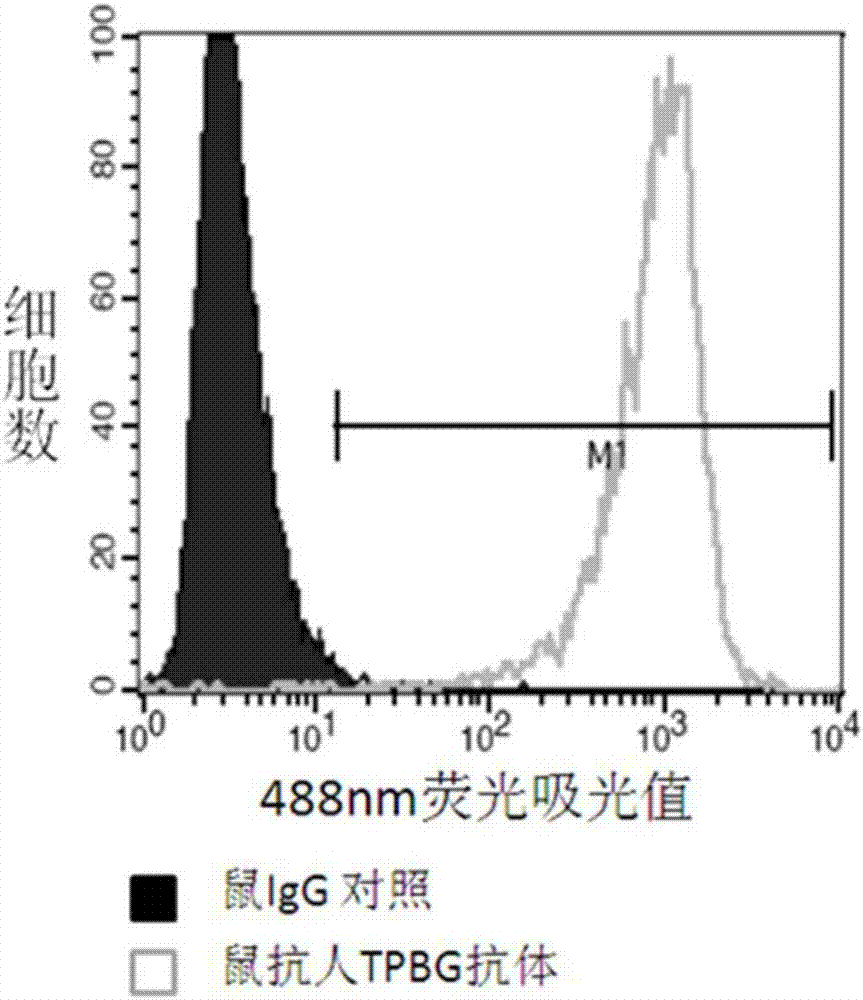
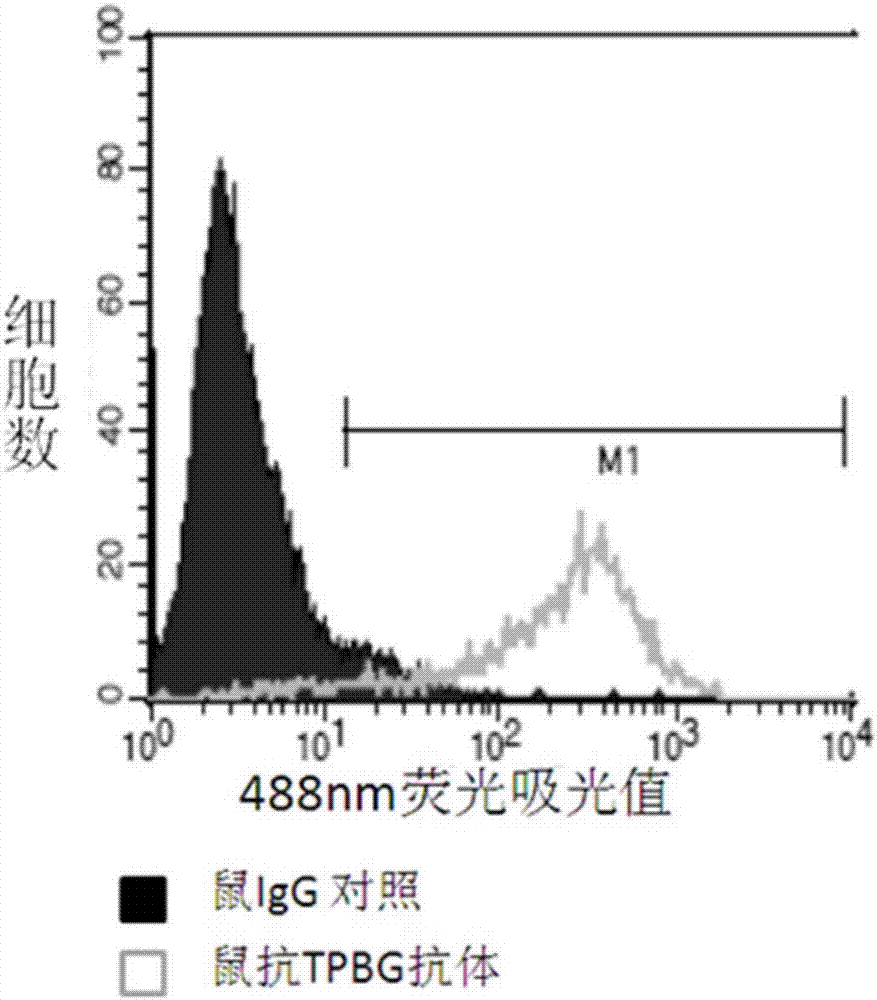
[0226] A. Enzyme-linked immunosorbent assay (ELISA) to detect the binding of TPBG antibody to TPBG protein
[0227] The purified TPBG antibody obtained in Example 2 was reacted with human TPBG-hFc protein (immunogen A).
[0228] Dilute the purified immunogen A obtained in Example 1 (see Example 1 step (1) for its preparation method) with PBS to a final concentration of 1.0 μg / mL, and then add 100 μL per well to a 96-well ELISA plate. Seal with plastic film Incubate overnight at 4°C, wash the plate twice the next day with plate washing solution [PBS containing 0.01% (v / v) Tween20], add blocking solution [containing 0.01% (v / v) Tween20 and 1% (w / w) PBS of BSA] blocked at room temperature for 2 hours. Pour off the blocking solution, add the purified TPBG antibody 100 μ L per well of the gained Example 2. After incubation for 2 hours at 37° C., wash the plate with the plate washing solution [containing 0.01% (v / v) Tween20 in P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com