Method of preparing ketolide macrolide antibiotics
A technology for macrolides and antibiotics, applied in the field of preparation, can solve the problems of difficult removal, low yield, unstable structure of carbonylimidazole, etc., achieve good product purity, high reaction yield, and avoid the effect of impurity formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The preferred embodiments of the present invention are given below in conjunction with the accompanying drawings to describe the technical solution of the present invention in detail.
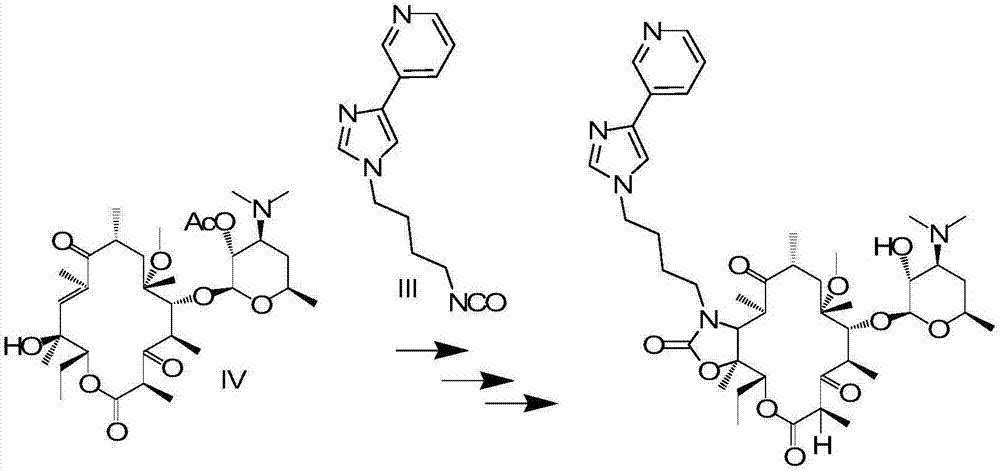
[0029] Such as figure 1 Shown, the present invention prepares the method for ketolide macrolide antibiotics and comprises the following steps:
[0030] Step 1, the side chain is first reacted with triphosgene to generate isocyanate, namely compound III;
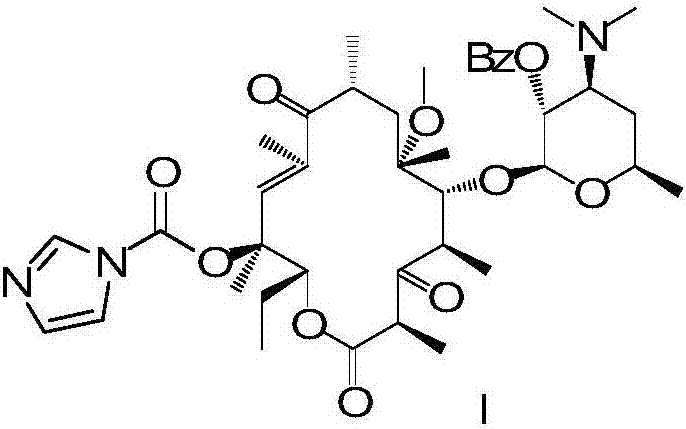
[0031] In step 2, the isocyanate obtained in step 1 is docked with intermediate IV, and cyclized to generate an oxazolone compound;
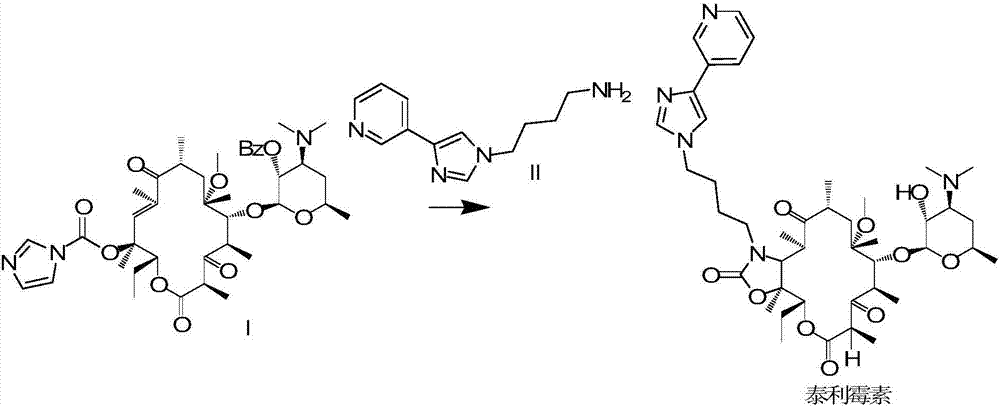
[0032] Step 3, removing the hydroxyl protecting group from the oxazolone compound obtained in Step 2 to obtain telithromycin.
[0033] Synthesis of Intermediate IV
[0034] In reaction bottle, add clarithromycin (50g, 0.067mol), triethylamine (18.75ml, 0.135mol, 2eq), ethyl acetate (350ml) stir and mix, add benzoic anhydride (22.5g, 0.1mol, 1.5eq), the addition was completed, and the reaction was stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com