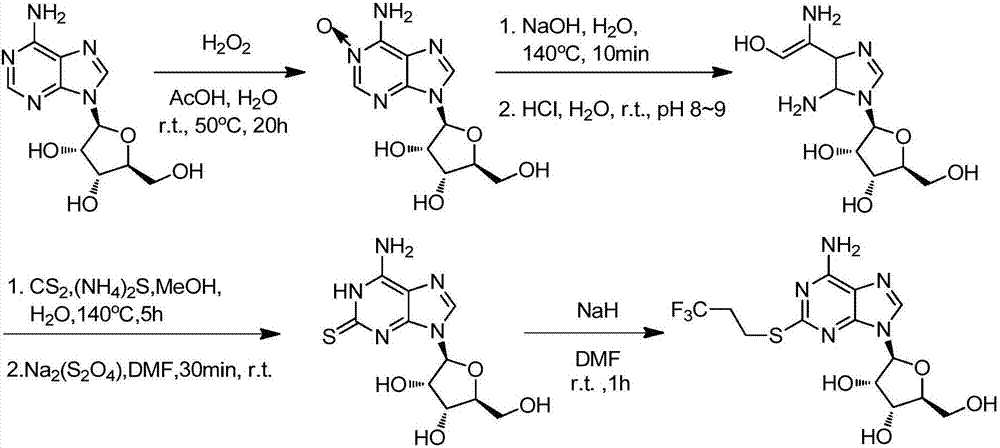
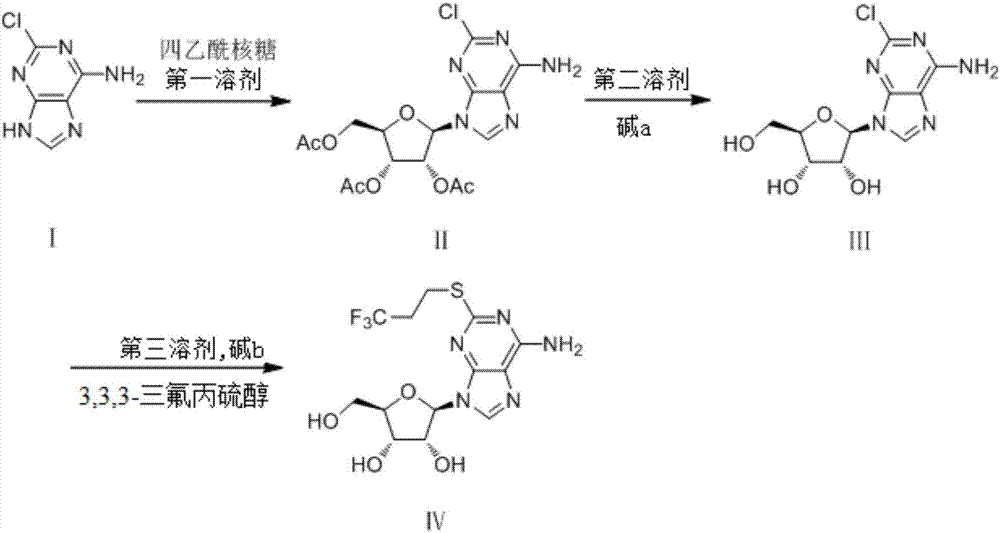
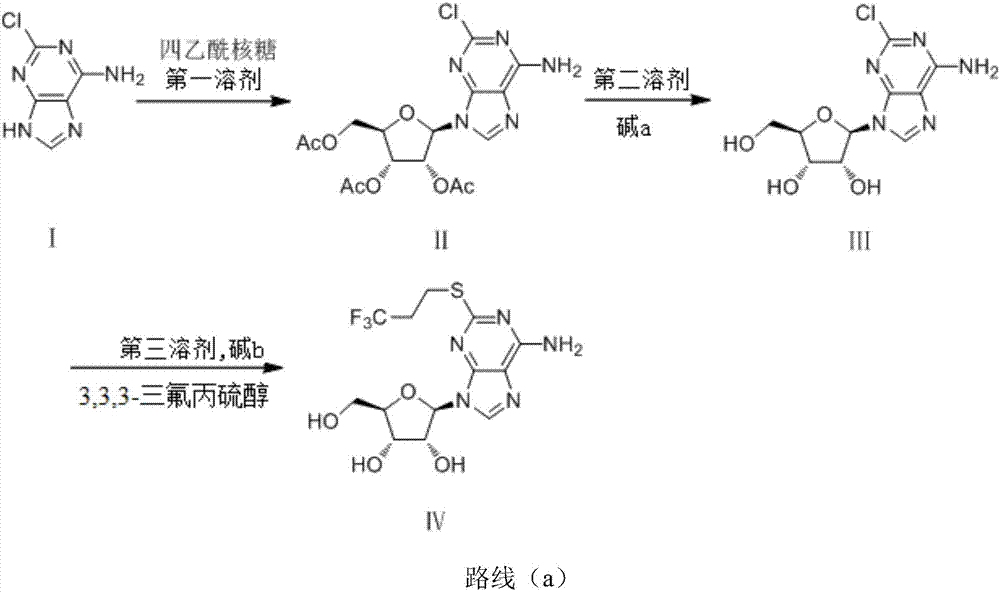
Synthetic method of 2-(3,3,3-trifluoropropylthio) adenosine
A technique for the synthesis of trifluoropropylthio, which is applied in the field of synthesis of 2-adenosine, which can solve the problems of unmonitorable hydrolysis reaction, low yield of ring-closing reaction, numerous reaction steps, etc., achieving low cost and simplified synthesis route , the effect of a simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0035] The preparation of embodiment 1.1 formula (II) compound
[0036] Under an ice-water bath, in a 50mL single-necked bottle, add 0.4g (1.0eq) of the raw material formula (I) 2-chloroadenine, 1.5g (2.0eq) of ribose, and 5mL of nitromethane, and the system becomes a yellow suspension , adding SnCl 4 0.62g (1.0eq), the temperature was slowly raised to room temperature, and the stirring was continued for 3 hours. Thin-layer chromatography detected that the reaction was complete.
[0037] Add 20 mL of ethyl acetate, add saturated aqueous sodium bicarbonate solution to wash, and dry the ethyl acetate, evaporate it to dryness and pass through the column (eluent: ethyl acetate:petroleum ether at 1:1) to obtain a light yellow solid compound of formula (II) 0.82g, yield 81%.
[0038] The NMR data are as follows:
[0039] 1 H NMR (400MHz, CDCl 3 )δ7.97(s,1H),6.75(s,2H),6.18(d,J=5.6Hz,1H),5.81(t,J=5.6Hz,1H),5.68–5.57(m,1H), 4.44(s,1H),4.41(s,2H),2.43(s,1H),2.15(d,J=6.1Hz,3H),2.1...
Embodiment 12
[0041] The preparation of embodiment 1.2 formula (II) compound
[0042] Under an ice-water bath, in a 50mL single-necked bottle, add 0.4g (1.0eq) of the raw material formula (I) 2-chloroadenine, add 1.5g (2.0eq) of ribose, add 5mL of tetrahydrofuran, the system is a yellow suspension, add SnCl 4 0.62g (1.0eq), the temperature was slowly raised to room temperature, and the stirring was continued for 3 hours. Thin-layer chromatography detected that the reaction was complete.
[0043] Add 20 mL of ethyl acetate, add saturated aqueous sodium bicarbonate solution to wash, and dry the ethyl acetate, evaporate it to dryness and pass through the column (eluent: ethyl acetate:petroleum ether at 1:1) to obtain the compound of formula (II) as light yellow solid 0.69g, yield 68%.
[0044] The NMR data are as follows:
[0045] 1 H NMR (400MHz, CDCl 3 )δ7.97(s,1H),6.75(s,2H),6.18(d,J=5.6Hz,1H),5.81(t,J=5.6Hz,1H),5.68–5.57(m,1H), 4.44(s,1H),4.41(s,2H),2.43(s,1H),2.15(d,J=6.1Hz,3H),2.13(d...
Embodiment 13
[0047] The preparation of embodiment 1.3 formula (II) compound
[0048] Under an ice-water bath, add 0.4g (1.0eq) of the raw material formula (I) 2-chloroadenine, 1.5g (2.0eq) of ribose, and 5mL of nitromethane into a 50mL single-necked bottle, and the system becomes a yellow suspension , adding SnCl 4 0.62g (0.5eq), the temperature was slowly raised to room temperature, and the stirring was continued for 3 hours. Thin-layer chromatography detected that the reaction was complete.
[0049] Add 20 mL of ethyl acetate, add saturated aqueous sodium bicarbonate solution to wash, and dry the ethyl acetate, evaporate it to dryness and pass through the column (eluent: ethyl acetate:petroleum ether at 1:1) to obtain the compound of formula (II) as light yellow solid 0.53g, yield 52.5%.
[0050] The NMR data are as follows:
[0051] 1 H NMR (400MHz, CDCl 3 )δ7.97(s,1H),6.75(s,2H),6.18(d,J=5.6Hz,1H),5.81(t,J=5.6Hz,1H),5.68–5.57(m,1H), 4.44(s,1H),4.41(s,2H),2.43(s,1H),2.15(d,J=6.1Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com