Method for preparing glucosyl group-alpha-cyclodextrin
A glucose-based and cyclodextrin technology, applied in the direction of fermentation, can solve problems such as increased branching rate, and achieve the effect of effective separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of glucosyl-alpha-cyclodextrin, said method comprising the following specific steps:
[0027] (1) Take 10 mg of maltodextrin in a 2 mL centrifuge tube, add 1 mL of pH 5.5 (20 mM) phosphate buffer solution, add 0.36 U of CGTase, and react at 60 ° C for 12 h;
[0028] (2) Insulate for 15 minutes in a water bath at 100°C;
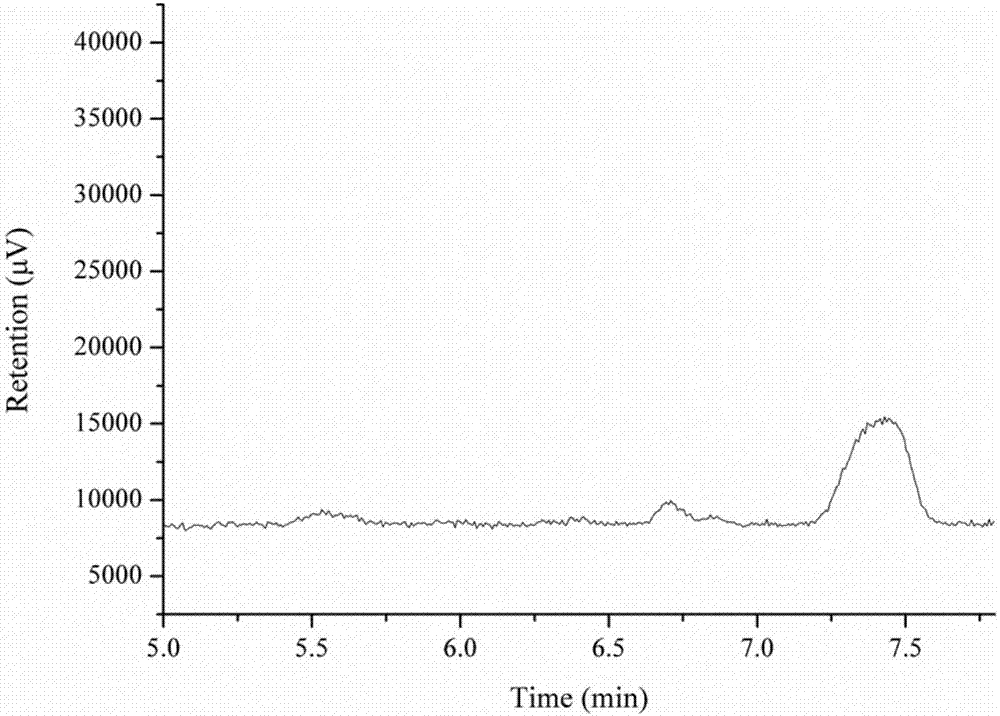
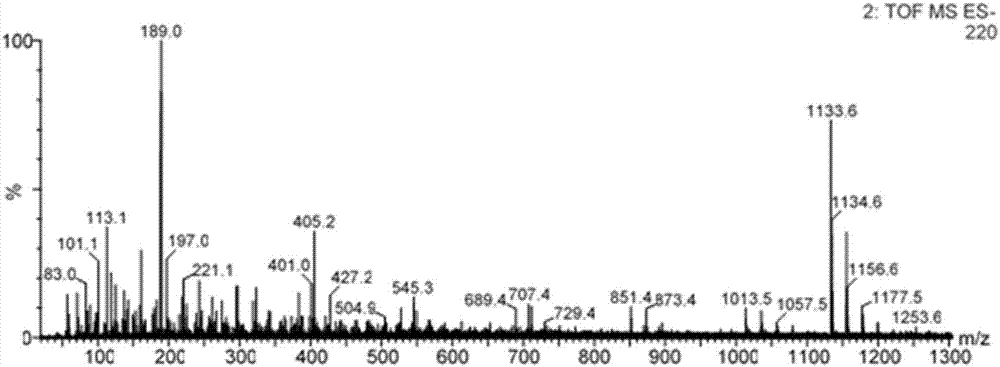
[0029] (3) Separating and purifying the product obtained in step (2) by using a high performance liquid phase method to finally obtain 0.19 mg of glucosyl-α-cyclodextrin.
Embodiment 2
[0031] A preparation method of glucosyl-alpha-cyclodextrin, said method comprising the following specific steps:
[0032] (1) Take 10 mg of maltodextrin in a 2 mL centrifuge tube, add 1 mL of pH 5.5 (20 mM) phosphate buffer solution, add 0.36 U of CGTase, and react at 60 ° C for 12 h;
[0033] (2) Add 0.72U glucoamylase to the reaction system, react for 12 hours under the reaction conditions of 45° C., and incubate at 100° C. for 15 minutes;
[0034] (3) Separating and purifying the product obtained in step (2) by using a high performance liquid phase method to finally obtain 0 mg of glucosyl-α-cyclodextrin.
Embodiment 3
[0036] A preparation method of glucosyl-alpha-cyclodextrin, said method comprising the following specific steps:
[0037] (1) Take 10 mg of maltodextrin in a 2 mL centrifuge tube, add 1 mL of pH 6.0 (20 mM) phosphate buffer solution, add 0.1 U of CGTase, and react at 60 ° C for 12 h;
[0038] (2) Heat in a water bath at 80°C for 25 minutes to make the enzyme activity of CGTase reach 0.001U;
[0039] (3) Add 0.5U glucoamylase to the reaction system, react for 12 hours under the reaction conditions of 45° C., and incubate at 100° C. for 15 minutes to prepare a mixture containing glucosyl-α-cyclodextrin;
[0040] (4) Separating and purifying the product obtained in step (3) by using a high performance liquid phase method to finally obtain 0.23 mg of glucosyl-α-cyclodextrin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com