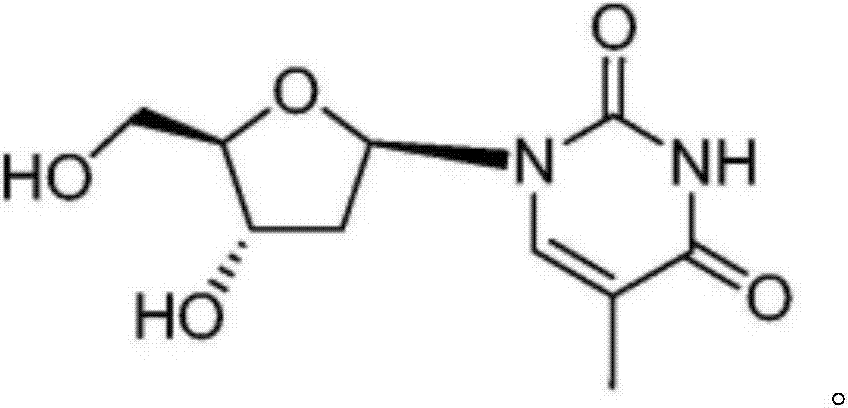
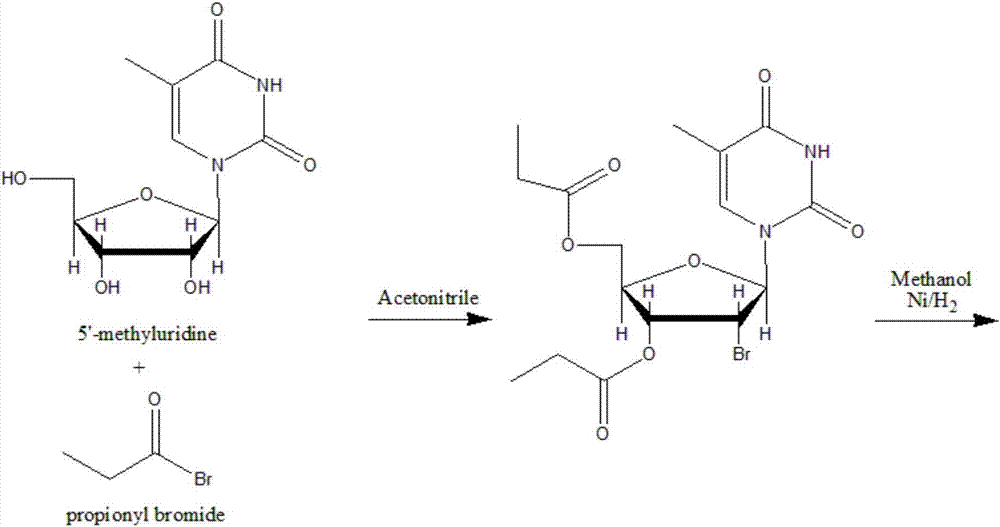
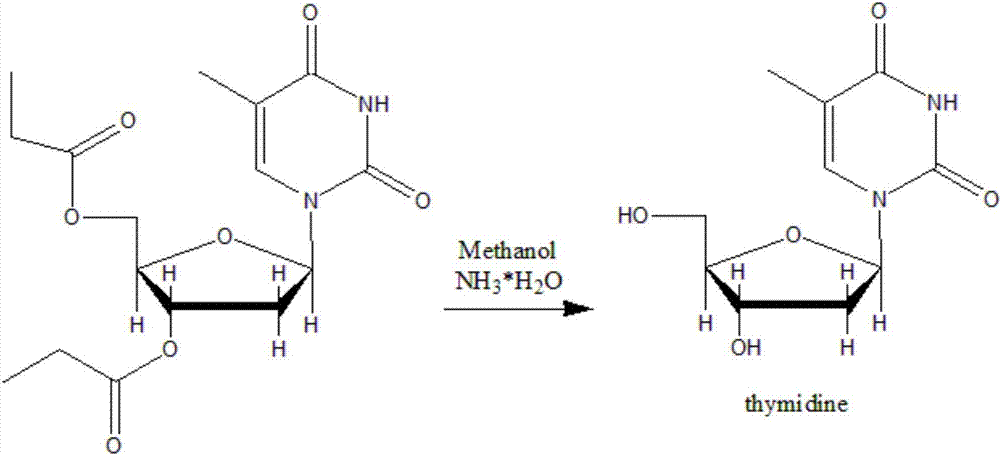
Synthetic method of beta-thymidine
A synthetic method and technology of thymidine, which is applied in the field of synthesis of β-thymidine, can solve problems such as the high cost of fermentation broth wastewater treatment, difficult treatment of bromine and nickel waste, and multiple industrial waste products, so as to achieve the removal of dangerous processes and shorten the production time. Chemical reaction steps, effects of reducing industrial waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 1eq 5-methyluridine, 0.1eq adenosine triphosphate (ATP), 0.1eq NADPH, enzyme concentration flavin adenine dinucleotide (FAD), enzyme concentration MgSO 4 , glucose dehydrogenase, uridine kinase, sepitate kinase, ribonucleoside diphosphate reductase, thymidylate kinase and thymidine kinase in phosphate buffer containing 10 mM NaCl, using HCl and NaOH to maintain pH The value is 6, the reaction temperature is 20°C, and the reaction time is 20h.
[0035] Compared with the common chemical synthesis method, the waste water generated in the reaction process is reduced by 33%.
Embodiment 2
[0037] Add 1eq 5-methyluridine, 0.1eq adenosine triphosphate (ATP), 0.1eq NADPH, enzyme concentration flavin adenine dinucleotide (FAD), enzyme concentration MgSO 4 , glucose dehydrogenase, uridine kinase, sepitate kinase, ribonucleoside diphosphate reductase, thymidylate kinase and thymidine kinase in phosphate buffer containing 10 mM NaCl, using HCl and NaOH to maintain pH The value is 7, the reaction temperature is 45°C, and the reaction time is 30h.
[0038] Compared with the common chemical synthesis method, the waste water generated in the reaction process is reduced by 31%.
Embodiment 3
[0040] Add 1eq 5-methyluridine, 0.1eq adenosine triphosphate (ATP), 0.1eq NADPH, enzyme concentration flavin adenine dinucleotide (FAD), enzyme concentration MgSO 4 , glucose dehydrogenase, uridine kinase, sepitate kinase, ribonucleoside diphosphate reductase, thymidylate kinase and thymidine kinase in phosphate buffer containing 10 mM NaCl, using HCl and NaOH to maintain pH The value is 8, the reaction temperature is 30°C, and the reaction time is 37h.
[0041] Compared with the common chemical synthesis method, the waste water generated in the reaction process is reduced by 38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com