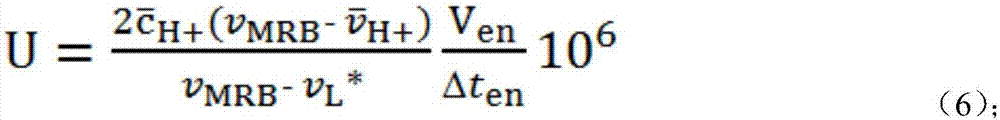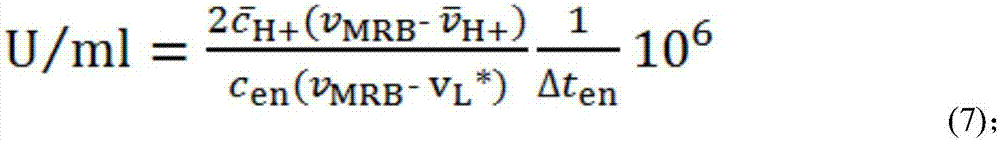Electrophoresis titration method for determining activity of peroxidase
A peroxidase, active technology, applied in the field of bioanalytical technology and medical testing, can solve the problems of low throughput, high cost, large sample consumption and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
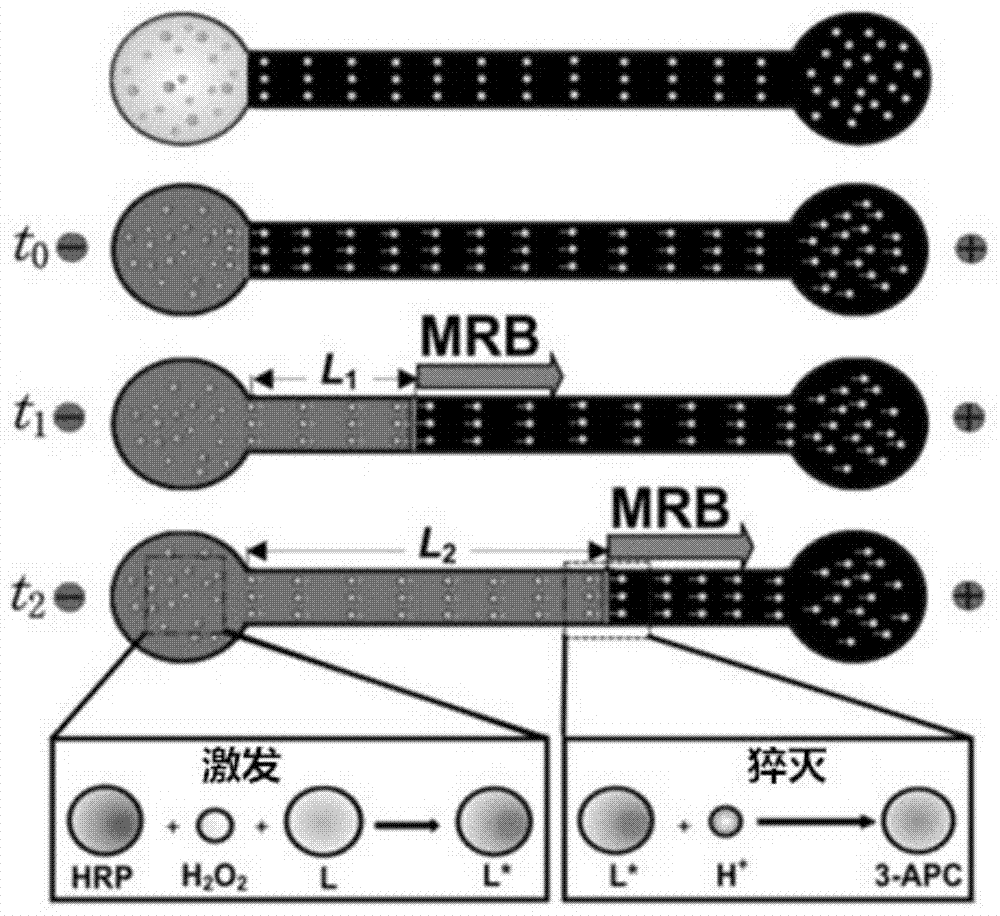
[0079] In the present embodiment, the horseradish peroxidase activity is determined by electrophoretic titration method, and the electrophoretic titration method is as follows: figure 1 shown. First, fill the channel part of the chip with gel, inject the mixed solution a of hydrochloric acid solution and background electrolyte solution into the anode pool, and inject the mixed solution b of chemiluminescent bottom solution and background electrolyte solution into the cathode pool; The oxidase solution is injected into the cathode cell to catalyze the reaction of the chemiluminescent base solution to generate the excited state luminol anion L* that emits blue fluorescence; after the enzyme-catalyzed reaction is stable for a period of time, a voltage is applied to make the excited state generated in the cathode cell The state luminol anion L* moves to the anode and interacts with the hydrogen ion H in the anode pool moving to the cathode + MRB is formed, and MRB moves to the an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com