Preparation method and application of oxygen vacancy containing manganese/sulfur dioxide composite
A technology of manganese dioxide and composite materials, applied in nanotechnology for materials and surface science, electrical components, battery electrodes, etc., to achieve good catalytic activity, clean and environmentally friendly preparation process, and reduce dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
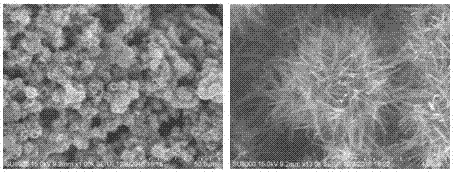
[0024] (1) Take 0.397g of potassium permanganate, dissolve it in 24mL of deionized water, stir for 30min, slowly add 6mL of 1mol / L dilute hydrochloric acid, continue stirring for 30min, transfer the solution to a 50mL polytetrafluoroethylene reaction kettle, 150℃ After hydrothermal heating for 6 hours, the obtained hydrothermal product was centrifuged and dried to obtain manganese dioxide;
[0025] (2) Take 0.1 g of the prepared manganese dioxide and heat it at 200°C for 2 hours under a hydrogen-argon mixed gas atmosphere to obtain manganese dioxide containing oxygen vacancies;
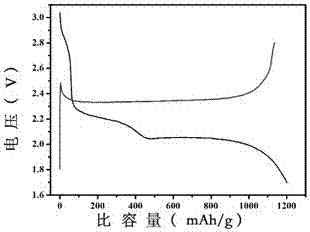
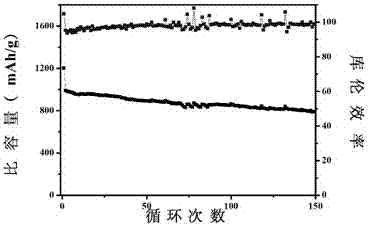
[0026] (3) Grind 50 mg of manganese dioxide containing oxygen vacancies and 200 mg of pure sulfur evenly, put them into a tube furnace, pass argon as a protective gas, and calcinate at 155°C for 12 hours to obtain MnO 2-x / S Composite.
Embodiment 2
[0028] (1) Take 0.397g of potassium permanganate, dissolve it in 24mL of deionized water, stir for 30min, slowly add 6mL of 1mol / L dilute hydrochloric acid, continue stirring for 30min, transfer the solution to a 50mL polytetrafluoroethylene reaction kettle, 150℃ After hydrothermal heating for 6 hours, the obtained hydrothermal product was centrifuged and dried to obtain manganese dioxide;
[0029] (2) Take 0.1 g of the prepared manganese dioxide and heat it at 200° C. for 2 hours in a hydrogen-argon mixed gas atmosphere to obtain manganese dioxide containing oxygen vacancies;
[0030] (3) Grind 50 mg of manganese dioxide containing oxygen vacancies and 200 mg of pure sulfur evenly, put them into a tube furnace, pass argon as a protective gas, and calcinate at 155°C for 12 hours to obtain MnO 2-x / S Composite.
Embodiment 3
[0032] (1) Take 0.397g of potassium permanganate, dissolve it in 24mL of deionized water, stir for 30min, slowly add 12mL of 1mol / L dilute hydrochloric acid, continue stirring for 30min, transfer the solution to a 50mL polytetrafluoroethylene reactor, 150℃ After hydrothermal heating for 6 hours, the obtained hydrothermal product was centrifuged and dried to obtain manganese dioxide;
[0033] (2) Take 0.1 g of the prepared manganese dioxide and heat it at 300° C. for 2 hours under a hydrogen-argon mixed gas atmosphere to obtain manganese dioxide containing oxygen vacancies;
[0034] (3) Grind 50 mg of manganese dioxide containing oxygen vacancies and 200 mg of pure sulfur evenly, put them into a tube furnace, pass argon as a protective gas, and calcinate at 155°C for 12 hours to obtain MnO 2-x / S Composite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com