Bisabolane sesquiterpene analogue, and preparation method and application thereof
A drug and reaction technology, used in pharmaceutical formulations, drug combinations, anti-tumor drugs, etc., can solve problems such as toxicity and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the preparation of formula I compound
[0049] in N 2Under protection, add 4-bromo-2-thiophenecarbaldehyde (382.1mg, 2.0mmol), p-cyanophenylboronic acid (353mg, 2.4mmol) and 1,4-dioxane (20mL) into a 50mL three-necked reaction flask After stirring and dissolving, cesium carbonate (1629.1mg, 5.0mmol) and water (10mL) were added, and stirring was continued for 5min at room temperature, and tetrakistriphenylphosphine palladium (115.6mg, 0.1mmol) was added to the reaction system. 2 Under protection, the reaction was continued under reflux for 6h. After the reaction solution was cooled to room temperature, dichloromethane (20 mL) was added and the layers were separated. The aqueous layer was extracted with dichloromethane (2×10 mL). The organic phases were combined and dried overnight over anhydrous sodium sulfate. After filtration, the solvent was evaporated to dryness under reduced pressure to obtain a crude product, which was separated by silica gel column...
Embodiment 2
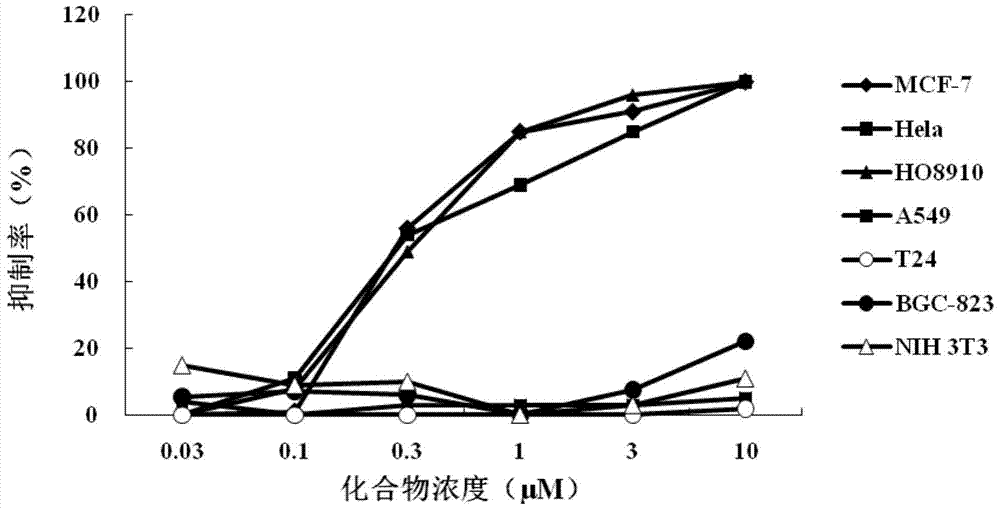
[0054] Embodiment 2, the effect of the compound shown in formula I on the proliferation and growth of tumor cells
[0055] Construction of cell screening models: breast cancer cells (MCF-7), cervical cancer cells (HeLa), ovarian cancer cells (HO8910), lung cancer cells (A549), bladder cancer cells (T24), gastric cancer cells (BGC-823) and small Mouse embryonic fibroblasts (NIH 3T3) were purchased from the Basic Medical Cell Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences.
[0056] MTT method (MTT colorimetric method) was used to test the effect of the compound shown in formula I prepared in Example 1 on the proliferation and growth of the above six tumor cells and NIH 3T3 cells. In order to verify the dose-response of several tumor cells to the compound, the compound shown in formula I prepared in Example 1 and the positive control drug cisplatin all use DMSO (dimethyl sulfoxide, Dimethyl sulfoxide, DMSO) as a solvent to prepare a concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com