3,4,5-trisubstituted-4,5-dihydroisoxazole, derivative and synthetic method and application thereof
A technology of dihydroisoxazole and three substitutions, applied in 3 and 4, can solve the problems of cumbersome steps and achieve the expected effect of simple reaction system, high product utilization value and market commercialization prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
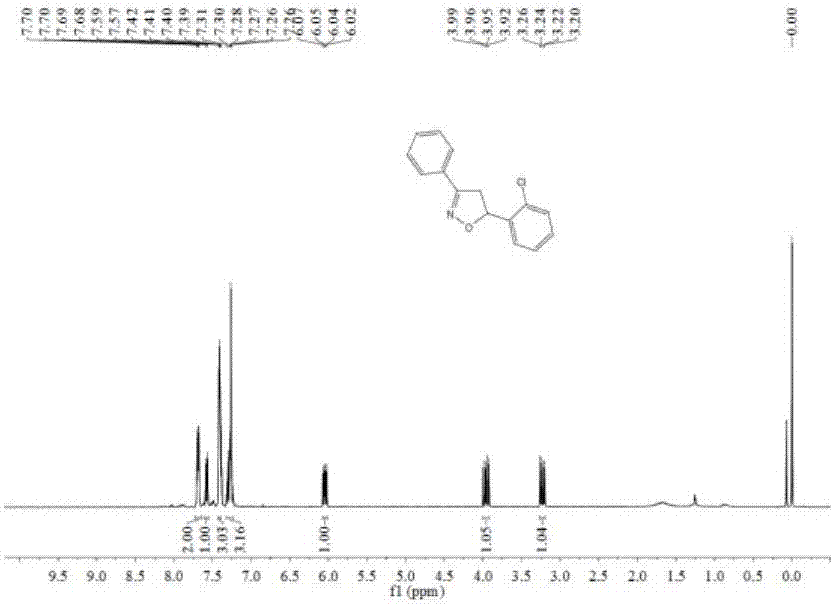
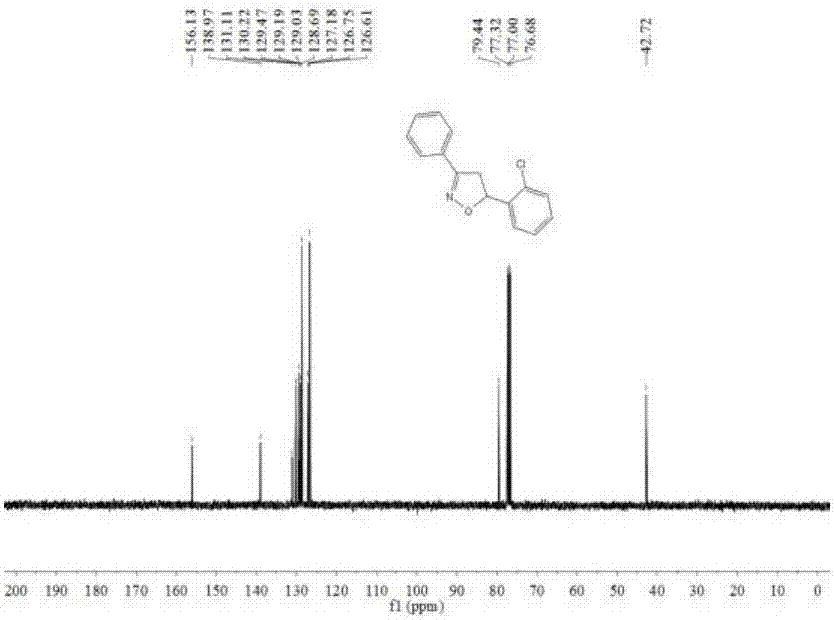
[0135] The NMR and mass spectrometry data of embodiment 1 product are as follows:
[0136] 1H NMR (400MHz, CDCl3) δ7.69 (dd, J = 6.6, 2.9Hz, 2H), 7.58 (d, J = 7.3Hz, 1H), 7.42–7.39 (m, 3H), 7.31–7.24 (m, 3H), 6.05(dd, J=11.1, 6.9Hz, 1H), 3.96(dd, J=16.8, 11.2Hz, 1H), 3.23(dd, J=16.8, 6.9Hz, 1H).13C NMR(101MHz, CDCl3)δ156.13,138.97,131.11,130.22,129.47,129.19,129.03,128.69,127.18,126.75,126.61,79.44,77.32,77.00,76.68,42.72. 258.06802,found258.06799.
Embodiment 2
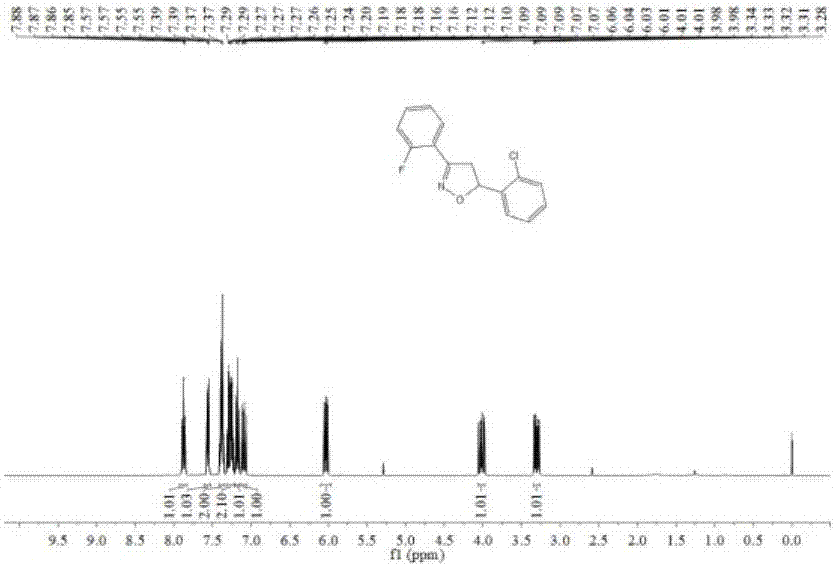
[0137] The NMR and mass spectrometry data of embodiment 2 product are as follows:
[0138] 1H NMR (400MHz, CDCl3) δ7.87 (td, J = 7.6, 1.6Hz, 1H), 7.56 (dd, J = 7.6, 1.6Hz, 1H), 7.41–7.35 (m, 2H), 7.31–7.22 ( m,2H),7.18(td,J=8.0,1.2Hz,1H),7.12–7.07(m,1H),6.03(dd,J=11.2,7.2Hz,1H),4.06–3.98(m,1H) ,3.34–3.27(m,1H).13C NMR(101MHz,CDCl3)δ160.27(d,J=252.9Hz),152.97(d,J=3.1Hz),138.78,131.84(d,J=8.6Hz) ,131.15,129.48,129.03,127.13,126.52,124.42(d,J=3.4Hz),117.28(d,J=11.6Hz),116.50,116.28,79.62(d,J=2.3Hz),77.32,77.00,76.68 ,44.26(d,J=6.8Hz).HRMS(ESI)m / z calcd for C15H12ClFNO+(M+H)+276.05860,found 276.05908.
Embodiment 3
[0139] The NMR and mass spectrum data of embodiment 3 product are as follows:
[0140] 1H NMR (400MHz, CDCl3) δ7.65–7.59(m,2H),7.41–7.23(m,6H),6.06(dd,J=11.0,7.1Hz,1H),4.08(dd,J=17.3,11.0 Hz,1H),3.36(dd,J=17.2,7.1Hz,1H).13C NMR(101MHz,CDCl3)δ156.11,138.53,132.79,131.15,130.89,130.52,130.47,129.48,129.07,128.62,127.190,126. 126.61,80.01,77.32,77.00,76.68,44.92.HRMS(ESI)m / zcalcd for C15H12Cl2NO+(M+H)+292.02905,found 292.02936.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com