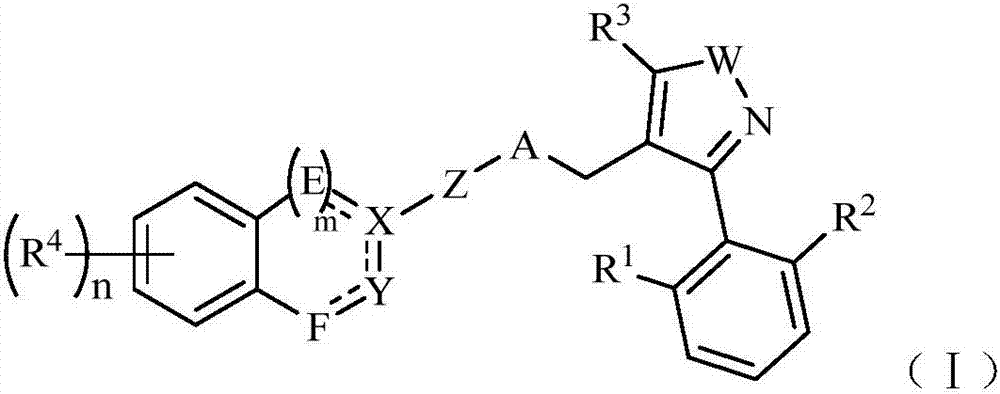
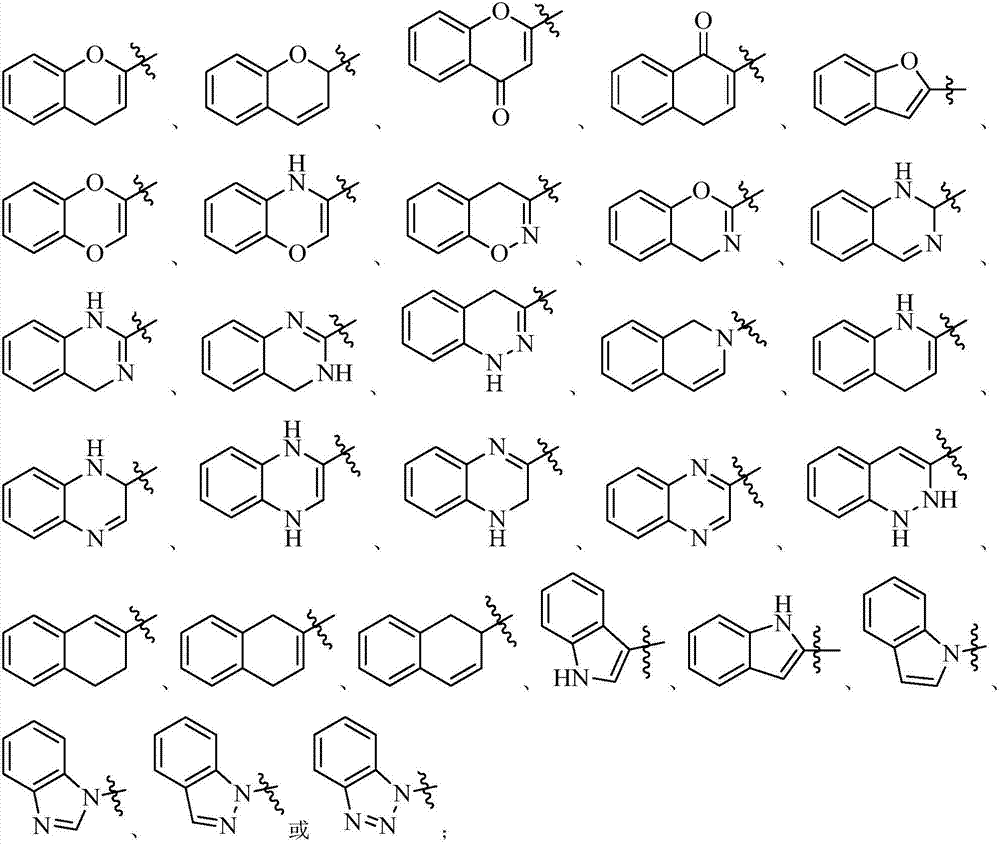
FXR agonist
A technology selected from, alkyl, applied in the field of FXR receptor agonists, which can solve the problems of low bioavailability and instability to light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0131] The present invention also provides a preparation method for the compound of formula (I), which includes but is not limited to the following process route (wherein, the definitions represented by each abbreviation are as follows: DCM: dichloromethane; DMSO: dimethyl sulfoxide; EA: Ethyl acetate; MeOH: methanol; NBS: N-bromosuccinimide; NCS: N-chlorosuccinimide; PE: petroleum ether; THF: tetrahydrofuran; DIBAL-H: diisobutyl hydrogenation aluminum; DMAP: 4-dimethylaminopyridine)
[0132] The specific exemplary steps are as follows:
[0133] 1. Preparation of Intermediate 1
[0134] Dissolve starting material 1 in an organic solvent (such as ethanol), slowly add starting material 2 in batches, after the addition is complete, add an alkaline solution (such as NaOH solution), heat to 60°C-90°C for 5-48 hours. After the reaction was completed, the solvent was removed from the reaction solution under reduced pressure, the solid was washed with water and dried to obtain inter...
experiment example 1
[0164] Experimental Example 1: In vitro biochemical analysis of compounds of the present invention
[0165] (1) Test substance: the compound of the present invention, its chemical name and preparation method are shown in the preparation examples of each compound.
[0166] (2) Experimental method:
[0167] Dissolve the detection compound in 100% DMSO, dilute 1000 times, take 160nL, then add 3.84μL detection buffer; Target / Antibody mixture, dilute 2 times, then add 8μL solution; add 4.0μL coactivator peptide diluted 4 times ; Incubate at room temperature for 60 minutes; After incubation, detect and analyze data on a fluorescent microplate reader.
[0168] (3) Experimental results and conclusions:
[0169] Table 1 Biochemical analysis of compounds of the present invention
[0170]
[0171] As can be seen from Table 1, the compound of the present invention has a certain stimulant effect on the FXR receptor, and has important effects on the treatment of related diseases such...
experiment example 2
[0172] Experimental example 2: Effects of the compounds of the present invention on the relative expression of BSEP mRNA in HepG2 cells and human hepatocytes
[0173] (1) Test substance: the compound of the present invention, its chemical name and preparation method are shown in the preparation examples of each compound.
[0174] Control drug: PX-104, see background technology for specific structure; PX-102, racemate of PX-104.
[0175] PBS: Phosphate buffered saline.
[0176] (2) Experimental method:
[0177] ①Laying cells, adding compounds and collecting cells
[0178] Use trypsin to digest and collect the cells, and measure the cell concentration; according to the counting results, resuspend the cells to a density of 7.5e5cell / mL; inoculate 2 mL of cells in each well of a 6-well cell culture plate; place the culture plate in an incubator, at 37°C, 5%CO 2 Conditioned for 24 hours.
[0179] Use DMSO to dilute the test compound to 12150, 4050, 1350, 450, 150, 50, 16.67,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com