HIV-1 (human immunodeficiency virus-1) integrase inhibitor tablet and preparation method thereof
An integrase inhibitor, HIV-1 technology, applied in pill delivery, antiviral agents, pharmaceutical formulations, etc., to achieve the effects of simple preparation process, high dissolution rate, and portability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
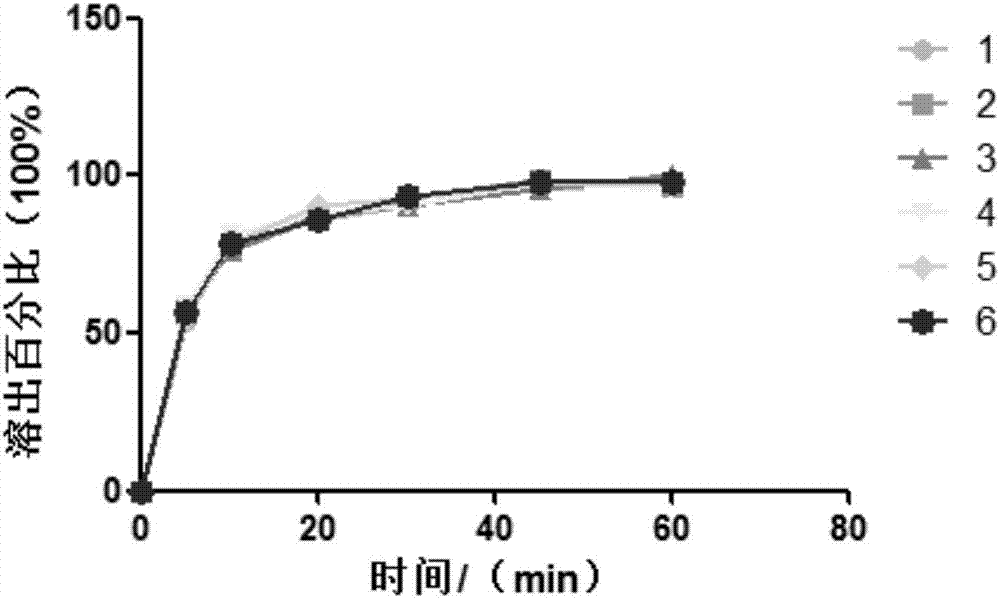
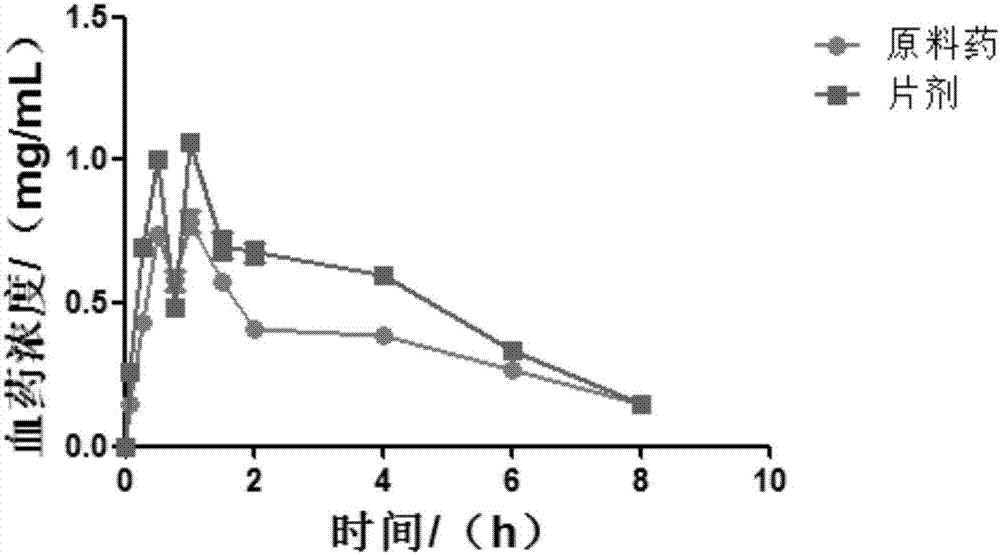
Image
Examples
preparation example Construction
[0023] The preparation method for preparing HIV-1 integrase inhibitor tablet provided by the invention comprises the following steps:
[0024] First, HIV-1 integrase inhibitor HIV-A5 is mixed with pharmaceutically acceptable auxiliary materials to obtain a drug mixture. In this step, the HIV-1 integrase inhibitor HIV-A5 and pharmaceutically acceptable auxiliary materials are first dried and sieved (usually 50-80 mesh, 60 mesh is used below), and then mixed , wherein the auxiliary material is a combination of one or more of fillers, disintegrants, binders and lubricants, therefore, preferably, by weight, 25 to 500 parts of HIV-1 integrase inhibitor HIV-A5, 50-600 parts of filler, 6-30 parts of disintegrating agent, 5-20 parts of binder and 1-6 parts of lubricant are mixed to obtain the drug mixture.
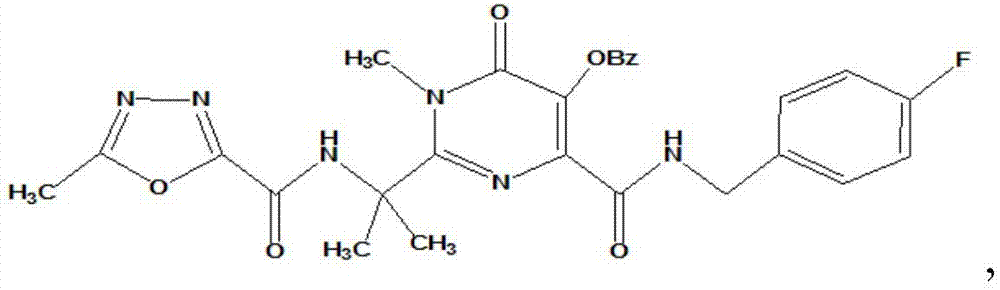
[0025] Wherein, the chemical formula of HIV-1 integrase inhibitor HIV-A5 is:
[0026] wherein, Bz represents a benzoyl group.
[0027] Wherein, the filler is a combination of...
Embodiment 1
[0034] Embodiment 1: the preparation of HIV-1 integrase inhibitor (HIV-A5) tablet
[0035]Sieve the HIV-1 integrase inhibitor and pharmaceutically acceptable excipients separately, weigh 300g of lactose, 10g of low-substituted hydroxypropyl cellulose, 10g of hydroxypropyl cellulose, 5g of magnesium stearate, mix, and then add 550 g of HIV-1 integrase inhibitor HIV-A were mixed; the above drug mixture was compressed into tablets, and the hardness of the HIV-1 integrase inhibitor tablet was controlled at 50N to obtain HIV-1 integrase inhibitor tablet. The HIV-1 integrase inhibitor tablet is packaged and labeled with polyoxyethylene blister + double-sided composite aluminum film bag.
Embodiment 2
[0036] Embodiment 2: the preparation of HIV-1 integrase inhibitor (HIV-A5) tablet
[0037] Sieve the HIV-1 integrase inhibitor and pharmaceutically acceptable auxiliary materials separately, weigh 150g of lactose, 150g of pregelatinized starch, 12g of crospovidone, 6g of hydroxypropyl cellulose, 5g of magnesium stearate, Mix, then add HIV-1 integrase inhibitor HIV-A5 50g and mix; The above drug mixture is compressed into tablets, and the hardness of the HIV-1 integrase inhibitor tablet is controlled at 60N to obtain HIV-1 integrase inhibitor tablet agent. The HIV-1 integrase inhibitor tablet is packaged and labeled with polyoxyethylene blister + double-sided composite aluminum film bag.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com