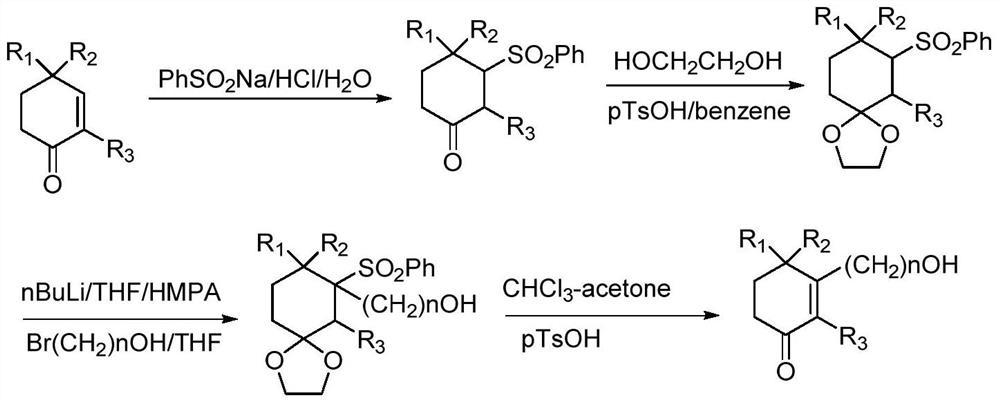
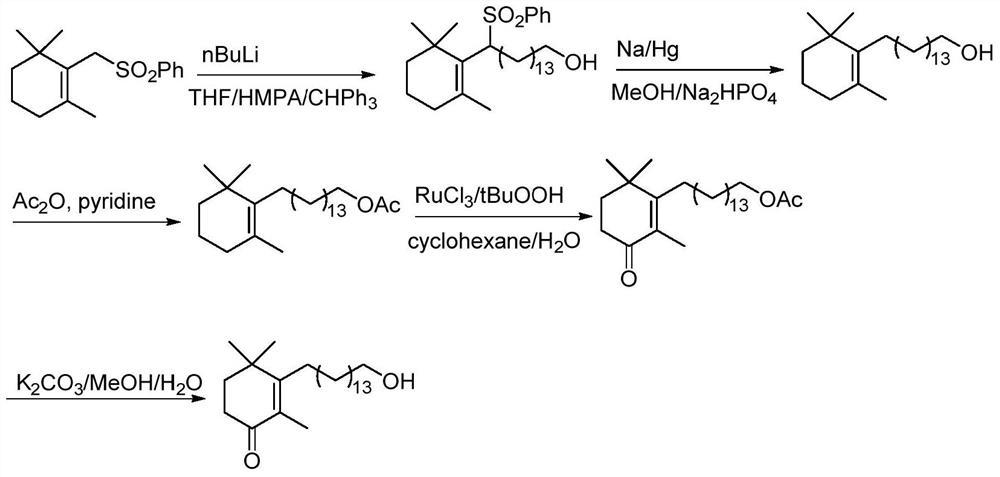
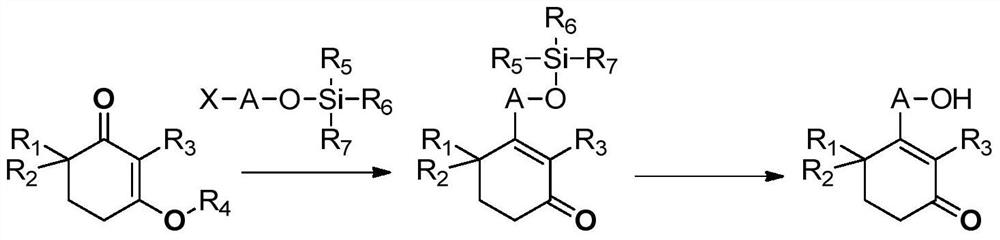
A kind of preparation method of high-purity cyclohexenone long-chain alcohol
A cyclohexenone long-chain alcohol and cyclohexenone technology, which is applied in the field of medicinal chemistry and synthetic chemistry, can solve the problems of high cost, difficult purification, and low melting point of column chromatography, and achieve avoidance of column chromatography and short route , good yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 13
[0084] Preparation Example 13-isobutoxy-2,6,6-trimethylcyclohex-2-en-1-one
[0085]
[0086] 2,4,4-Trimethylcyclohexyl-1,3-dione VII (80g, 1eq) and isobutanol (76.9g, 2eq) were added to cyclohexane (400mL), and p-TSA· H 2 O (5g, 0.05eq), heated and refluxed to separate water for 16h. After-treatment, cooled to ambient temperature, washed successively with 5% sodium hydroxide (80 mL), water (80 mL) and saturated brine (80 mL), dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 3-isobutoxy-2 ,6,6-Trimethylcyclohex-2-en-1-one (103.65 g, 95%). 1H NMR (400MHz, CDCl3): δ3.77 (d, 2H, J=6.4Hz), 2.55-2.58 (m, 2H), 1.95-2.05 (m, 1H), 1.82 (t, 2H, J=6.4Hz) ), 1.72(s, 3H), 1.11(s, 6H), 1.01(d, 6H, J=6.4Hz).
[0087] Preparation Example 23-cyclohexylmethoxy-2,6,6-trimethylcyclohex-2-en-1-one
[0088]
[0089] 2,4,4-Trimethylcyclohexyl-1,3-dione VII (10g, 1eq) and cyclohexylmethanol (14.8g, 2eq) were added to cyclohexane (100mL), and p-TSA· H2O (0.62g, 0....
preparation example 43-
[0093] Preparation Example 43-Methoxy-2,6,6-trimethylcyclohex-2-en-1-one
[0094]
[0095] 2,4,4-Trimethylcyclohexyl-1,3-dione VII (2.7g, 1eq) and trimethyl orthoformate (2.8g, 1.5eq) were added to methanol (40mL), p- TSA·H2O (167 mg, 0.05 eq) was stirred at room temperature overnight. After-treatment, dichloromethane (30 mL) was added to dilute, washed successively with 5% sodium hydroxide (20 mL), water (10 mL) and saturated brine (10 mL), dried over anhydrous sodium sulfate, concentrated to dryness and purified by column to obtain 3 -Methoxy-2,6,6-trimethylcyclohex-2-en-1-one (2.19 g, 74.4%). 1 HNMR (400MHz, CDCl 3 ): δ3.81(s, 3H), 2.55-2.58(m, 2H), 1.95-2.05(m, 1H), 1.82(t, 2H, J=6.4Hz), 1.72(s, 3H), 1.11( s, 6H).
[0096] Preparation Example 53,3'-(propyl-1,2-dioxo)-bis(2,6,6-trimethylcyclohexyl-2-en-1-one)
[0097]
[0098] 2,4,4-Trimethylcyclohexyl-1,3-dione VII (5g, 1eq), 1,3-propanediol (1.23g, 0.5eq), p-TSA·H2O (311mg, 0.05eq) and toluene (30 mL) were add...
preparation example 93-(1
[0109] Preparation Example 93-(15-Chloropentadecyloxy)-2,6,6-trimethylcyclohexyl-2-en-1-one
[0110]
[0111] 2,4,4-Trimethylcyclohexyl-1,3-dione VII (1.3 g, 1.1 eq) and 15-chloropentadecanol VIII-1 (2 g, 1 eq) were added to cyclohexane (50 mL) Add p-TSA·H2O (72mg, 0.05eq), heat and reflux for 16h, post-treatment, cool to ambient temperature, use 5% sodium hydroxide (20mL), water (10mL) and saturated brine ( 10 mL) washed, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 3-(15-chloropentadecyloxy)-2,6,6-trimethylcyclohexyl-2-en-1-one (2.46 g, 80.9 %). 1 H NMR (400MHz, CDCl 3 ):δ3.97(t,2H,J=6.8Hz),3.45(m,2H,J=6.8Hz),2.54-2.55(m,2H),1.78-1.84(m,4H),1.68(s, 3H), 1.39-1.41(m, 4H), 1.22-1.35(m, 21H), 1.08(s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com