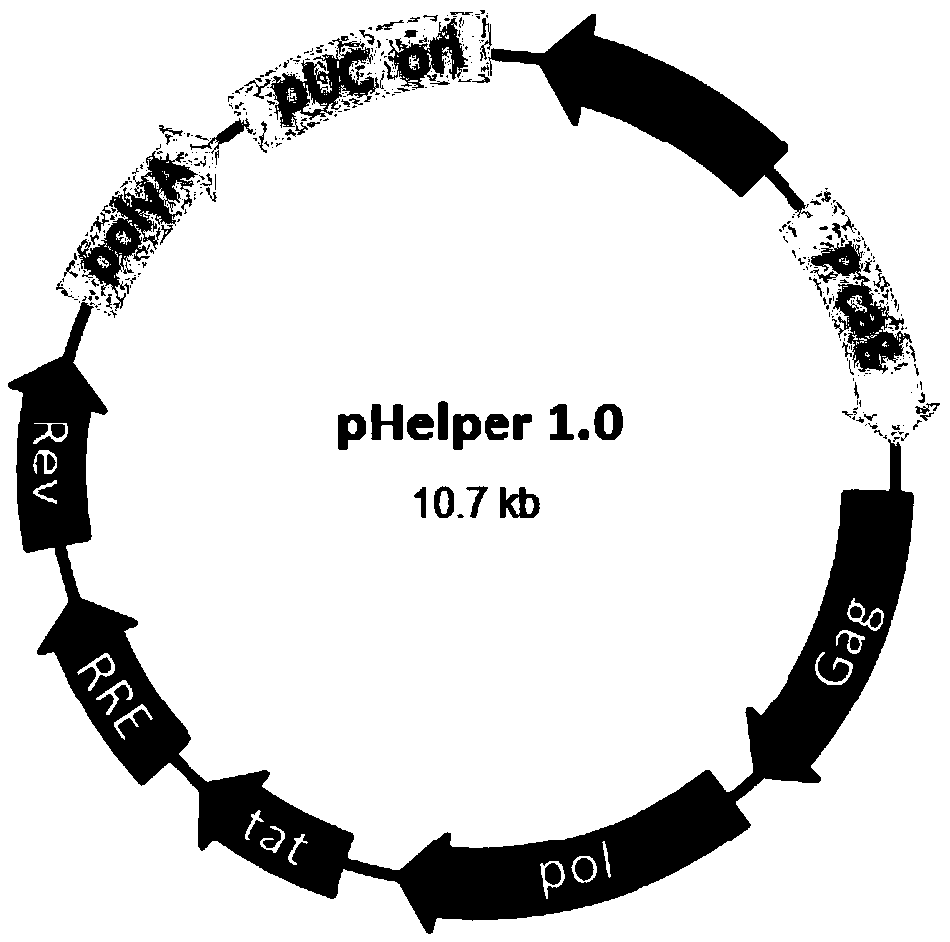
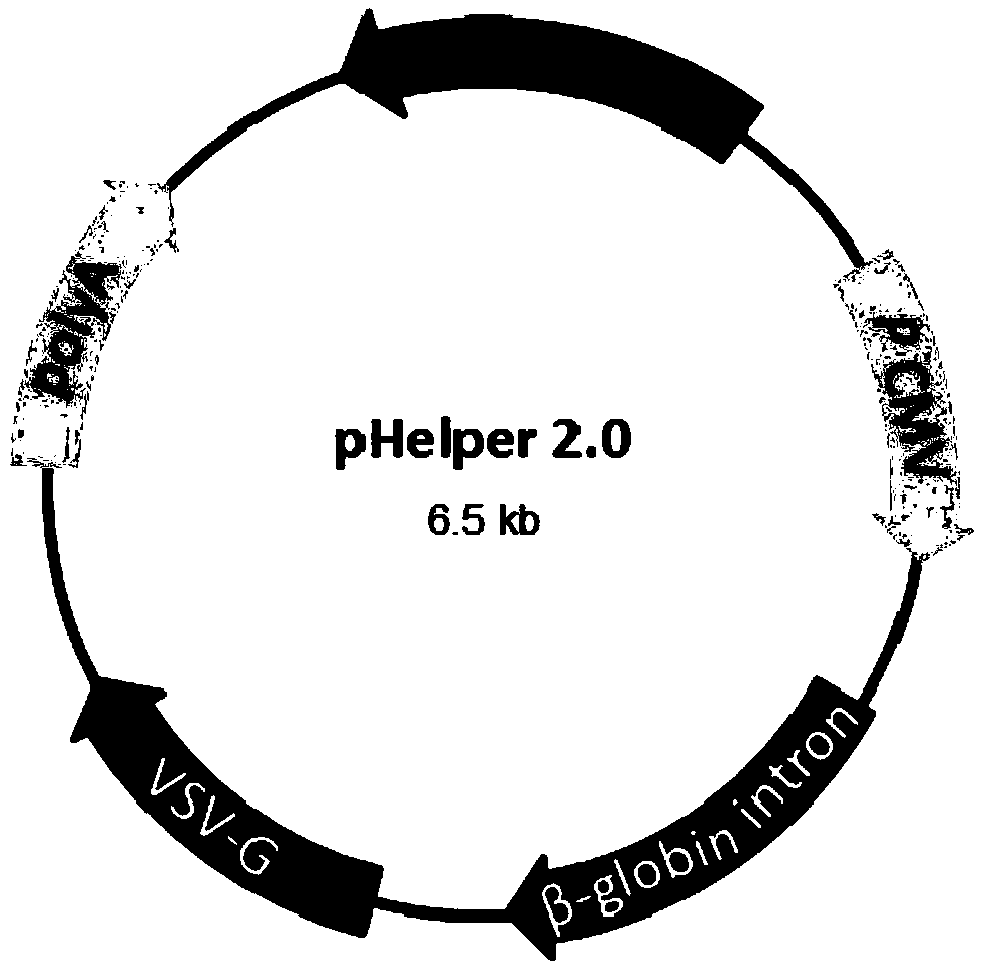
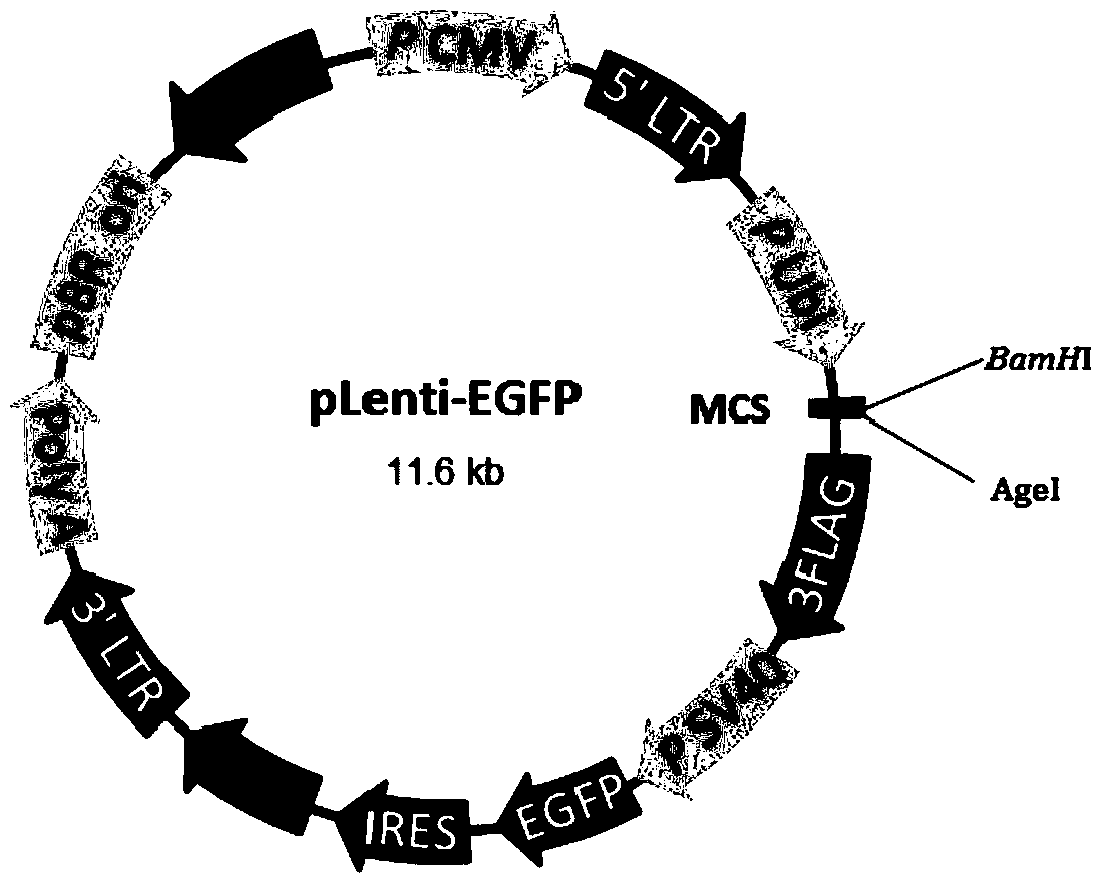
A method of preparing a lentivirus vector
A technology of lentiviral vectors and vector plasmids, applied in the biological field, can solve the problems of small host range of lentiviral products, and achieve the effects of reducing transduced cell death, good gene expression or RNAi interference, and stable expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of cGMP grade lentiviral vector particles comprises the following steps:
[0048] (1) Culture of HEK293T cells:
[0049] The production of lentiviral vector particles is in the 10-layer CellFactory (6320cm 2 , NUNC). HEK293T cells were divided into 2 × 10 per well 5 / cm 2 The density was inoculated in DMEM medium containing 10% FBS, and placed at 37°C, 5% CO 2 The culture was carried out in an environment with saturated humidity, and the transfection was carried out 3 days after inoculation.
[0050] (2) Transfection of HEK293T cells:
[0051] Before transfection, prepare 580 ml of DMEM medium containing 2% FBS, and add chloroquine to a final concentration of 20-30 mmol / L. Prepare calcium phosphate transfection system: Add 5865 μg DNA plasmid (pHelper 1.0: pHelper2.0: pLenti-EGFP=15:12:24) to 5ml Opti-MEM in sequence, then add 5750 μl of 2.5M CaCl 2 , and finally 60ml of 2x HBS was added dropwise on a stirring shaker. After the transfection syste...
Embodiment 2
[0058]The lentiviral vector particles prepared in Example 1 were tested for activity titer and physical titer, nucleic acid residue detection, serum protein residue detection, LDH detection, RCL detection, sterility detection and mycoplasma detection.
[0059] (1) Activity titer detection:
[0060] HEK293T cells were divided into 2 × 10 per well 3 Cells were seeded in 96-well plates and cultured in DMEM medium containing 10% FBS. After 24 hours of cell plating, the lentiviral vector particle products I, II, III, and IV obtained in different purification process steps were respectively transduced into cells according to the optimal MOI value. For the four groups of samples I, II, III, and IV, five consecutive 10-fold serial dilutions were performed with DMEM medium containing 10% FBS (MOI=10). And add one well of untransduced empty cells as a control for each group of samples. After 24 hours of transduction, 100 μl of DMEM medium containing 10% FBS was supplemented to each w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com