Preparation method of 6-bromopyridine-3-carboxaldehyde
A technology of bromopyridine and bromomethylpyridine is applied in the field of preparation of 6-bromopyridine-3-carbaldehyde, and can solve the problems of low conversion rate, unsuitability for industrial application, incomplete reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
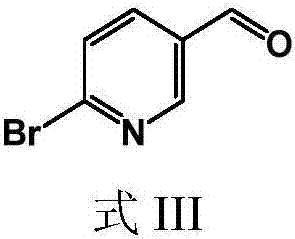
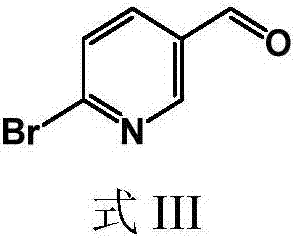
[0044] The present invention provides a preparation method of 6-bromopyridine-3-carbaldehyde, the structural formula of the 6-bromopyridine-3-carbaldehyde is shown in formula III:
[0045]
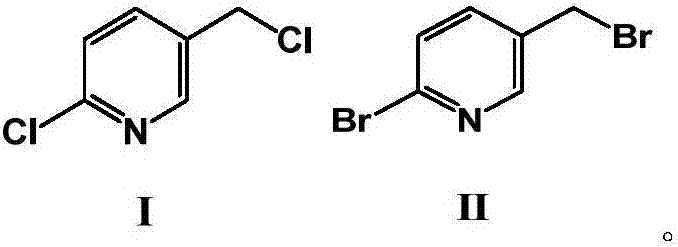
[0046] The preparation method comprises the steps of: carrying out a bromination reaction of 2-chloro-5-chloromethylpyridine (compound of formula I) in the presence of a brominating reagent to prepare 2-bromo-5-bromomethylpyridine ( Formula II compound), the structural formula of shown formula I compound and formula II compound is as follows:
[0047]
[0048] In the preparation method provided by the present invention, the bromination reagent can be various commonly used bromination reagents in the art, such as hydrogen bromide, bromine, dibromohydantoin, sodium bromide, phosphorus oxybromide, One or more combinations of N-bromosuccinimide, etc. In some preferred embodiments of the present invention, the bromination reagent may preferably be one or more combinations of hydrogen bro...
Embodiment 1
[0066] Take 100g of raw material 2-chloro-5-chloromethylpyridine, put it into Hastelloy autoclave, and add 800g of 25% hydrobromic acetic acid solution. Close the reaction kettle, stir and heat up to 100-110°C. React for 10 hours. The filtrate was removed by filtration and dried to obtain a crude product with a mass of 139.7 g and a content of 92%.
[0067]Dissolve 7.5g of sodium ethylate in 40g of ethanol, under nitrogen protection, and heat up to 50-60°C. 10 g of 2-nitropropane was added dropwise. After the dropwise addition, keep warm at 60°C for 1h. Take 20 g of the above-mentioned 2-bromo-5-bromomethylpyridine, dissolve it in 60 g of ethanol, add it dropwise into the system at 65°C, and keep it warm until the reaction is completed. After the reaction, the salt was removed by filtration. Ethanol was recovered by distillation, and 30 g of DCM was added to the residue, and the pH was adjusted to 6-7 with 1% hydrobromic acid, and the mixture was separated and washed. Th...
Embodiment 2
[0069] Take 100g of raw material 2-chloro-5-chloromethylpyridine, put it into a Hastelloy autoclave, and add 800g of 25% hydrobromic formic acid solution. Close the reaction kettle and raise the temperature to 100-110°C. React for 10 hours. The filtrate was removed by filtration and dried to obtain a crude product with a mass of 142.5 g and a content of 88%.
[0070] Take 11g of sodium tert-butoxide, dissolve it in 40g of solvent tert-butanol, protect it with nitrogen, and raise the temperature to 50-60°C. 10 g of 2-nitropropane was added dropwise. After the dropwise addition, keep warm at 65°C for 1h. Take 20 g of the above-mentioned 2-bromo-5-bromomethylpyridine, dissolve it in 60 g of tert-butanol, add it dropwise into the system at 65°C, and keep it warm until the reaction is completed. After the reaction was completed, the salt was removed by filtration. Recover tert-butanol, add 30 g of DCM to the residue, use 1% hydrobromic acid to pH=6-7, and separate and wash. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com