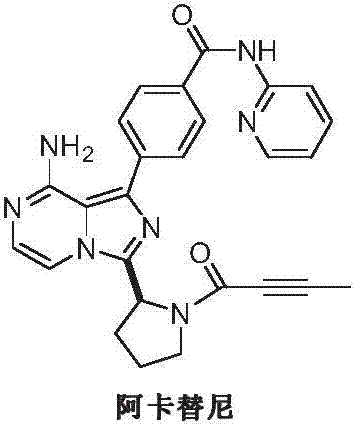
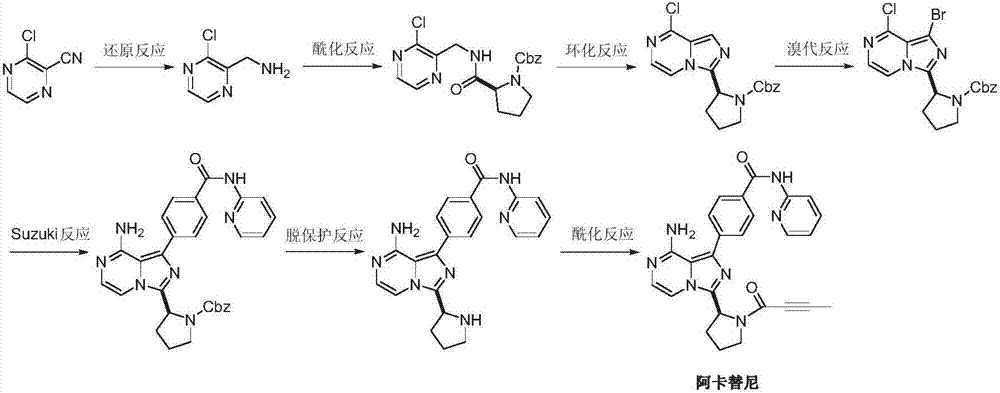
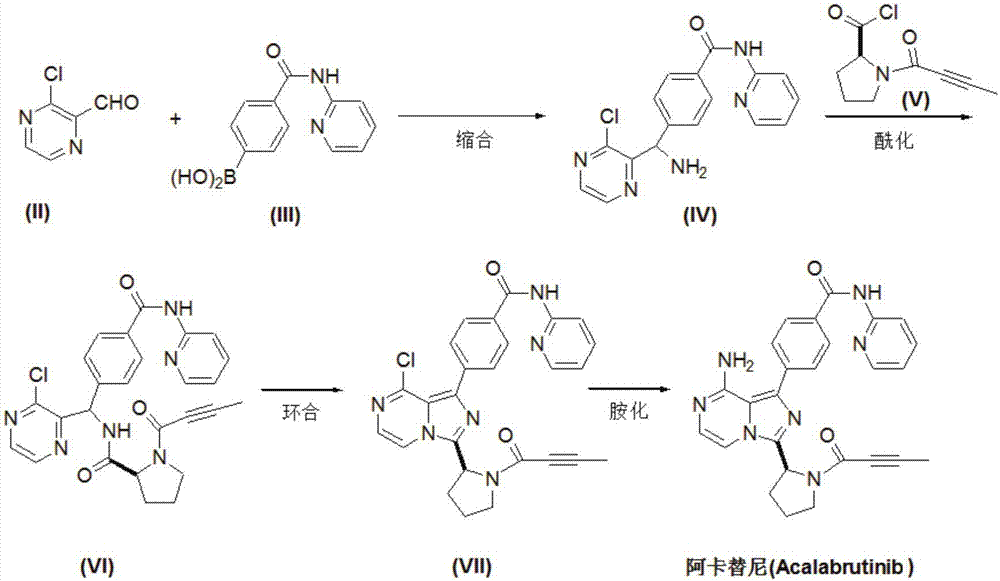
Preparation method of acalabrutinib
An acalatinib and a pyrazine-based technology, which is applied in the field of preparation of the leukemia-treating drug acalatinib, can solve the problems of many reaction steps, difficult industrialization, low total yield and the like, and achieves a simple process, environmental protection and economy. , the effect of promoting use and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The technical solutions involved in the present invention will be described in further non-limiting detail below in conjunction with preferred embodiments.
[0027] One embodiment of the present invention provides a preparation method of Acalabrutinib that can be used for the treatment of leukemia, comprising the following steps:
[0028] Step S1:
[0029] Add 3-chloro-2-formaldehyde pyrazine (II) (0.71g, 5mmol) and dioxane (20mL) into the pressure reactor, and feed ammonia gas (1.7g, 0.1mol) under stirring, then add 4-(Pyridin-2-yl-aminocarbonyl)phenylboronic acid (III) (2.42 g, 10 mmol), rhodium acetylacetonate dicarbonyl (0.26 g, 1 mmol) and water 4 mL. Seal the reactor, gradually raise the temperature to 80-90 degrees, react for 16-18 hours, TLC detection, the reaction is complete. Concentrate under reduced pressure, dissolve the residue with dichloromethane, wash with saturated sodium bicarbonate and water successively, and dry over anhydrous sodium sulfate. Con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com