Refining method of marbofloxacin
A refining method and technology of marbofloxacin, applied in the direction of organic chemistry, etc., can solve the problems affecting bioavailability, solubility, pharmacological safety, irregular crystal form, non-crystalline form, etc., to improve bioavailability and pharmacological safety. properties, improve purity and content, improve appearance and color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Add 10g of crude marbofloxacin to a 250ml three-necked flask, then add 45ml of water, stir at room temperature until there are no obvious particles, then add 2.2g of formic acid, and stir for 20min. Suction filtration to collect filter cake.
[0023] (2) Add 60ml of isopropanol and 20ml of water into a 250ml three-necked flask, then add the filter cake to suspend the filter cake in the mixed solvent, then add DIEA to adjust the pH to 8.0, stir to dissolve, add activated carbon, and heat up to reflux at 60°C 20min-40min, filter and collect the filtrate
[0024] (3) Add the filtrate to a 250ml three-neck flask, slowly heat up, and start to concentrate. When the temperature reaches 83°C, naturally cool down to 25°C, continue to cool down and crystallize to 8°C, continue to keep warm for 20 minutes, suction filter, wash, vacuum 60 After drying at ℃, 8.53 g of marbofloxacin fine product was obtained.
Embodiment 2
[0026] (1) Add 10g of crude marbofloxacin to a 250ml three-necked flask, then add 50ml of water, stir at room temperature until there are no obvious particles, then add 3.9g of acetic acid, and stir for 20min. Suction filtration to collect filter cake.
[0027] (2) Add 75ml of ethanol and 26.5ml of water into a 250ml three-necked flask, then add the filter cake, suspend the filter cake in the mixed solvent, then add ammonia water to adjust the pH to 8.5, stir to dissolve, add activated carbon, heat up and reflux at 60°C for 20min -40min, filter, collect filtrate
[0028] (3) Add the filtrate to a 250ml three-neck flask, slowly heat up, and start to concentrate. When the temperature reaches 81°C, naturally cool down to 20°C, continue to cool down and crystallize to 5°C, continue to keep warm for 20 minutes, filter, wash, and vacuum 60°C After drying at ℃, 9.02 g of marbofloxacin fine product was obtained.
Embodiment 3
[0030] (1) Add 10g of crude marbofloxacin to a 250ml three-neck bottle, then add 50ml of water, stir at room temperature until there are no obvious particles, then add 3g of formic acid, and stir for 20min. Suction filtration to collect filter cake.
[0031] (2) Add 70ml of methanol and 25ml of water into a 250ml three-necked flask, then add the filter cake to suspend the filter cake in the mixed solvent, then add triethylamine to adjust the pH to 8.5, stir to dissolve, add activated carbon, and heat up to reflux at 60°C 20min-40min, filter and collect the filtrate
[0032] (3) Add the filtrate to a 250ml three-necked flask, slowly heat up, and start to concentrate. When the temperature reaches 75°C, naturally cool down to 20°C, continue to cool down and crystallize to 5°C, continue to keep warm for 20 minutes, filter, wash, and vacuum 60°C ℃ drying, to obtain 8.13 grams of marbofloxacin fine.
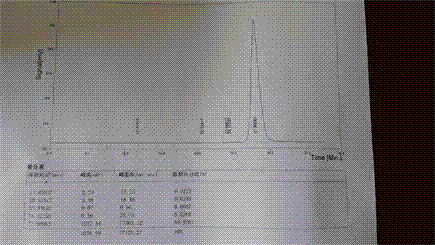
Description of drawings
[0033] figure 1 It is the high performance liquid c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com