Preparation method for Fe-N-C oxygen reduction catalyst
A catalyst and solvent technology, applied in the field of fuel cell catalytic materials, can solve problems such as difficulty in maintaining the stability of catalytic current, unstable graphitized C structure, and reduced catalyst activity, so as to reduce the probability of corrosion or oxidation, low equipment requirements, The effect of hindering reunion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 0.1g of P123 into 30ml of absolute ethanol, and disperse by ultrasonic. Then add 1.0 g of dicyandiamide and 0.34 g of ferric chloride hexahydrate, ultrasonically disperse it evenly, then raise the temperature to 50° C. and continue stirring until the solvent evaporates and becomes dry. The above dried precursor mixture was placed in a porcelain boat, heated to 250 °C under an inert atmosphere, kept for 1 h, then raised to 800 °C, kept for 2 h, and cooled to room temperature naturally. Then it was placed in 0.5M sulfuric acid solution, continuously stirred at 80°C for 8h, naturally cooled to room temperature, washed repeatedly with deionized water and then vacuum-dried to obtain a Fe-N-C catalyst with a special structure.
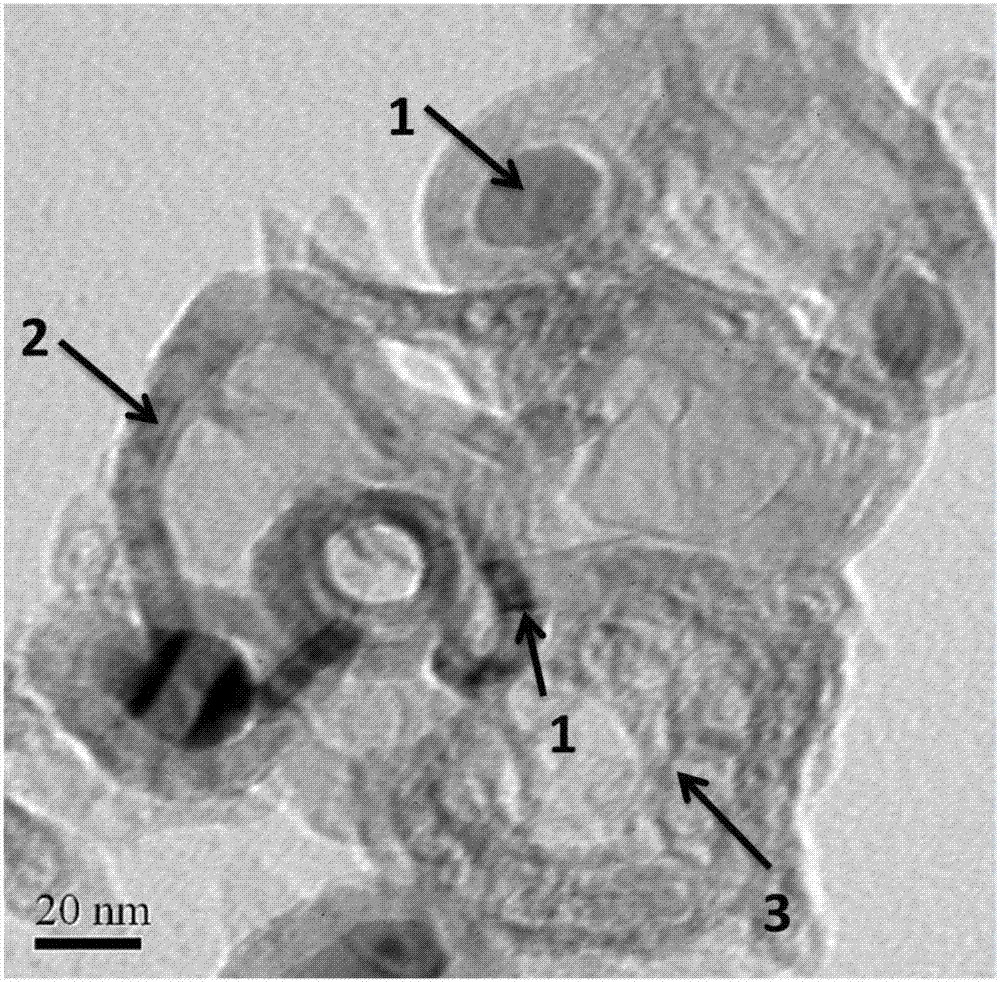
[0024] from figure 1 It can be seen that the Fe-N-C oxygen reduction catalyst prepared in the present invention is a composite structure in which iron nanoparticles are encapsulated in nitrogen-doped ultra-thin graphene nanosheets and curled carb...
Embodiment 2
[0026] Add 0.8g of P123 into 60ml of deionized water and ultrasonically disperse. Then add 2.0 g of melamine and 0.5 g of ferric sulfate heptahydrate, ultrasonically disperse it evenly, then raise the temperature to 80° C. and continue stirring until the solvent evaporates and becomes dry. The above-mentioned dried precursor mixture was placed in a porcelain boat, heated to 400°C under an inert atmosphere, kept for 0.5h, then raised to 900°C, kept for 3h, and cooled to room temperature naturally. Then it was placed in 6.0M sulfuric acid solution, continuously stirred at 60°C for 6h, naturally cooled to room temperature, washed repeatedly with deionized water and then vacuum-dried to obtain a Fe-N-C catalyst with a special structure.
Embodiment 3
[0028] Add 2.0g of F127 into 50ml of ethylene glycol and disperse by ultrasonic. Then add 4.0 g of urea and 1.0 g of ferric acetate tetrahydrate, ultrasonically disperse it evenly, then raise the temperature to 60° C. and continue stirring until the solvent evaporates and becomes dry. The above dried precursor mixture was placed in a porcelain boat, heated to 450°C under an inert atmosphere, kept for 2h, then raised to 950°C, kept for 1h, and cooled to room temperature naturally. Then it was placed in 5.0M hydrochloric acid solution, continuously stirred at 70°C for 10 h, cooled to room temperature naturally, washed repeatedly with deionized water and then vacuum-dried to obtain a Fe-N-C catalyst with a special structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com