Functionalized beta-sheet peptide stabilized membrane proteins, constructs comprising same, and methods of forming and using same
A membrane protein, functionalization technology, applied in chemical instruments and methods, organic chemistry methods, animal/human proteins, etc., can solve problems such as difficulty in scale, difficulty in production, and unstable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
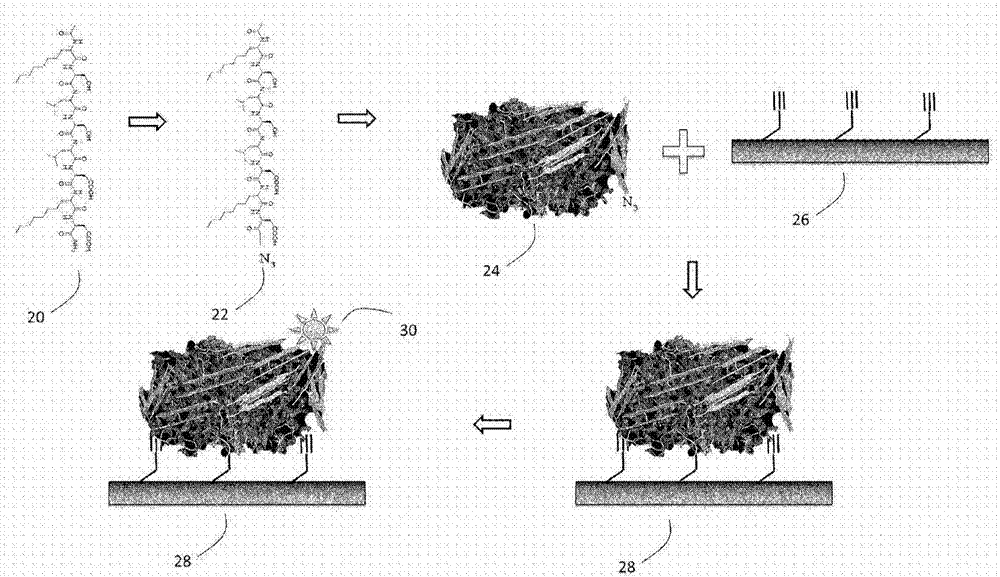
[0100] Example 1.0 - Preparation of functionalized β-sheet peptide (FBP)
[0101] The chemical synthesis of FBP on embodiment 1.1-solid-phase peptide synthesizer
[0102] Using amino acids with propargyl side chains, the inventors were able to synthesize functionalized beta-sheet peptides with alkyne functionality at the C-terminus on a solid-phase peptide synthesizer. Alkyne-FBP self-assembles into filamentous structures when diluted in phosphate-buffered saline, suggesting that alkyne-FBP retains the β-sheet formation propensity of β-sheet peptides. The synthesized alkyne-functionalized β-sheet peptide has the structure of BP1 but with a C-terminal propargylglycine, i.e., acetyl-(octyl)Gly-(octyl)Gly-Ser-Leu-Ser-Leu-Asp-( Octyl)Gly-Asp-(propargyl)Gly-NH 2 , (SEQ ID NO: 12). Self-assembly of alkyne-functionalized β-sheet peptides is shown in Figure 4A middle. Figure 4B A mass spectrum showing the correct molecular mass of high purity propargyl-BP1 is shown.
[0103]...
Embodiment 12
[0104] Example 1.2 - Confirmation of Alkyne Functionality on FBP1
[0105] In order to confirm the presence and active function of the alkyne group on the alkyne-BP1 synthesized in Example 1.1, the inventors performed a reaction to react the alkyne group on the peptide with fluorescent tetrathyrhodamine azide (Click-iT kit, LifeTechnology). Click to react. The inventors then ran the reaction products on an HPLC equipped with a fluorescence detector. As expected, additional fluorescent peaks not belonging to the kit components appeared on the elution curve (at Figure 5 Indicated by arrows in the upper panel of ; the HPLC profiles of individual kits and individual dyes are shown in Figure 5 middle and bottom panels). Therefore, the inventors could assign this peak to the dye-labeled alkyne-functionalized β-sheet peptide.
Embodiment 2
[0106] Example 2.0 - Membrane protein purification and BP:MP complex formation
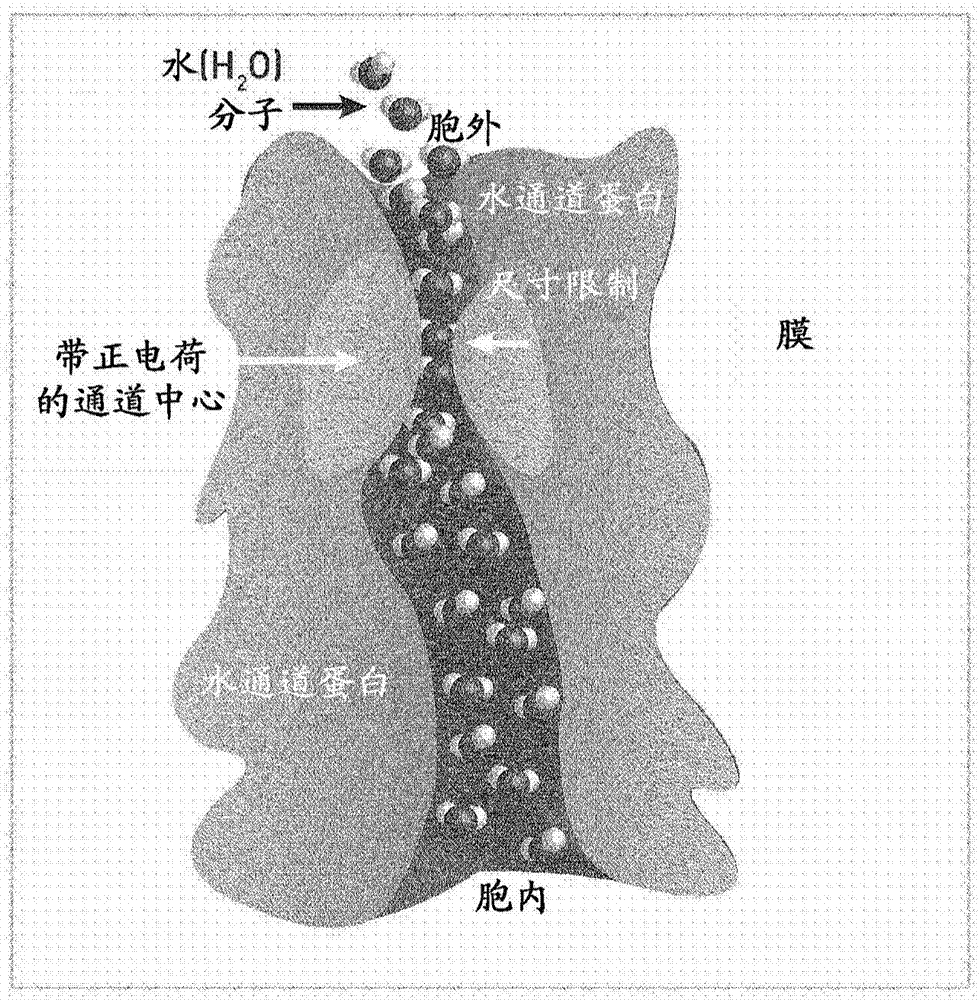
[0107] The inventors have purified aquaporin (AqpZ) and verified its function. AqpZ is an aqueous channel on the periplasmic membrane of Escherichia coli. AqpZ selectively transfers water at high rates and based on an osmotic gradient across the cell membrane. As noted above, existing attempts to use aquaporins in water purification applications have not been entirely successful. However, it is believed that FBP:AqpZ complexes according to the present disclosure will overcome at least some of the disadvantages associated with previous attempts.
[0108] According to standard molecular biology procedures, the inventors have (7) Overexpression and purification of recombinant AqpZ in Escherichia coli were optimized using n-dodecyl β-D-maltoside (DDM) as a solubilizing detergent. The gene encoding AqpZ was cloned using the plasmid pTrc10HisAqpZ described in literature (7) as a template. A small...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com