Application of 3-acetylthiotetronic acid derivatives in fungicides for controlling plant pathogens
A technology of acetylthiotetraketonic acid and plant pathogenic bacteria, which is applied in the field of pesticides and achieves the effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
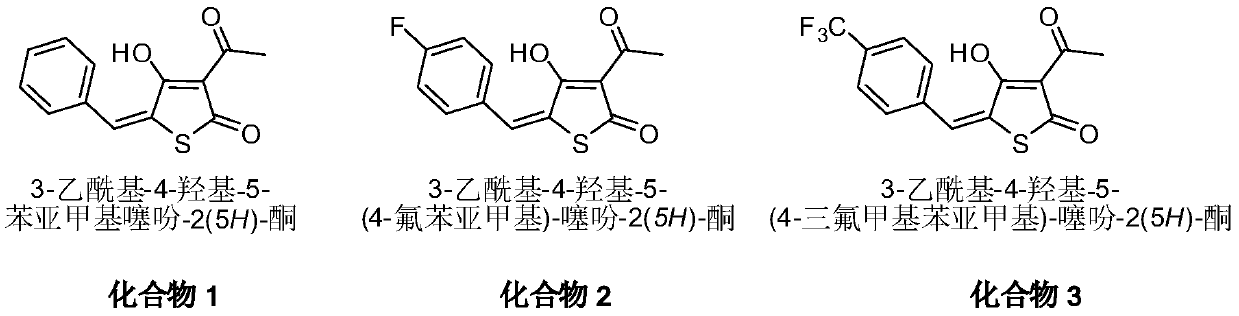
[0022] Dissolve 158mg of 3-acetylthiotetraketonic acid in 50ml of toluene, add 271mg of benzaldehyde and 20mg of p-toluenesulfonic acid, heat and reflux for 10 hours, phenomenon: light yellow gradually disappears, cool to room temperature. The toluene solvent was evaporated by rotary evaporation, and the product was purified by using ethyl acetate and methanol as recrystallization solvents with a yield of 42%. Mp: 151-152°C. 1 H NMR (600MHz, DMSO-d 6 )δ: 7.81(s, 1H), 7.65(d, J=7.6Hz, 2H), 7.50(t, J=7.5Hz, 2H), 7.45(t, J=7.3Hz, 1H), 2.47(s, 3H), 13 C NMR (150MHz, DMSO-d 6 )δ: 195.85, 187.05, 186.03, 134.20, 131.13(2C), 130.90, 130.73, 129.67(2C), 127.31, 108.18, 26.68; MS[ESI]: m / z([M-H] - ),245.0643;
[0023]
[0024] 3-Acetyl-4-hydroxy-5-benzylidenethiophen-2(5H)-one was obtained.
Embodiment 2
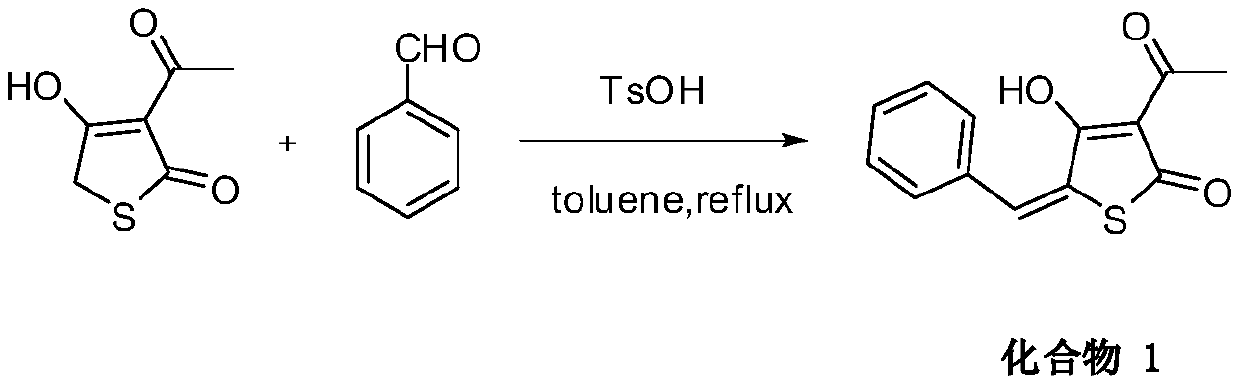
[0026] Dissolve 158 mg of 3-acetylthiotetraketonic acid in 50 ml of toluene, add 290 mg of p-fluorobenzaldehyde and 20 mg of p-toluenesulfonic acid, heat and reflux for 10 hours, phenomenon: light yellow gradually disappears, and cool to room temperature. The toluene solvent was evaporated by rotary evaporation, and the product was purified by using ethyl acetate and methanol as recrystallization solvents with a yield of 53%. Mp: 181-183°C. 1 H NMR (600MHz, DMSO-d 6 )δ: 7.79(s, 1H), 7.71(dd, J=8.6, 5.6Hz, 2H), 7.35(t, J=8.8Hz, 2H), 2.46(s, 3H). 13 C NMR (150MHz, DMSO-d 6 )δ: 195.56, 186.86, 186.03, 164.02, 162.36, 133.46, 131.00, 129.35, 127.38, 116.89, 116.74, 107.98, 26.84; MS[ESI]: m / z([M-H] - ),263.0349;
[0027]
[0028] 3-Acetyl-4-hydroxy-5-(4-fluorobenzylidene)thiophen-2(5H)-one was obtained.
Embodiment 3
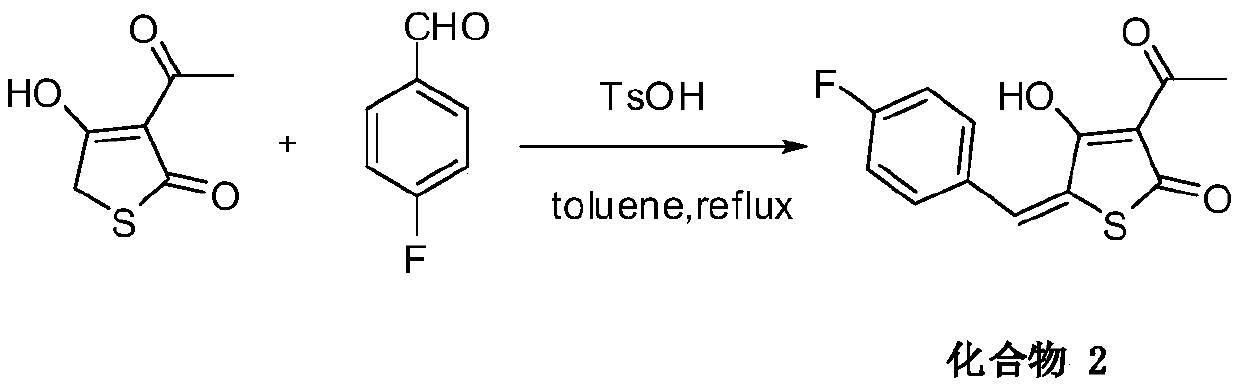
[0030] Dissolve 158 mg of 3-acetylthiotetraketonic acid in 50 ml of toluene, add 346 mg of p-trifluoromethylbenzaldehyde and 20 mg of p-toluenesulfonic acid, heat and reflux for 10 hours, phenomenon: light yellow gradually disappears, cool to room temperature . The toluene solvent was evaporated by rotary evaporation, and the product was purified by using ethyl acetate and methanol as recrystallization solvents with a yield of 55%. Mp: 162-164°C. 1 H NMR (600MHz, DMSO-d 6 )δ:7.84–7.79(m,4H),7.71(s,1H),2.40(s,3H). 13 C NMR (150MHz, DMSO-d 6 )δ: 193.76, 186.49, 186.01, 138.92, 132.80 131.08(2C), 126.27, 126.25(2C), 126.02, 109.98, 107.19, 27.54; MS[ESI]: m / z([M-H] - ), 314.0224;
[0031]
[0032] 3-Acetyl-4-hydroxy-5-(4-trifluoromethylbenzylidene)thiophen-2(5H)-one was obtained.
[0033] Three kinds of 3-acetylthiotetratonic acid derivatives 1-3 of the present invention are described as follows to the inhibition rate of apple rot fungus, corn leaf spot fungus, wheat hea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com