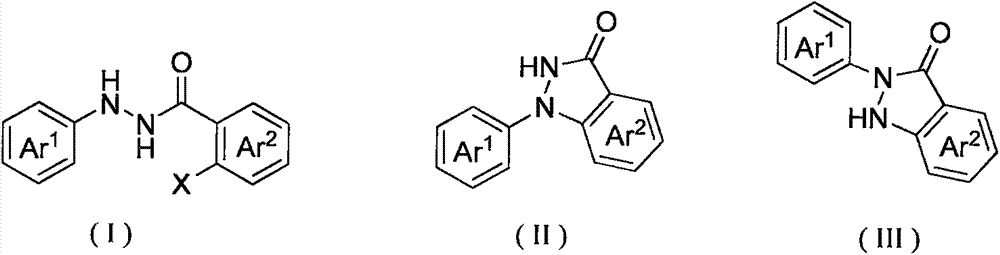
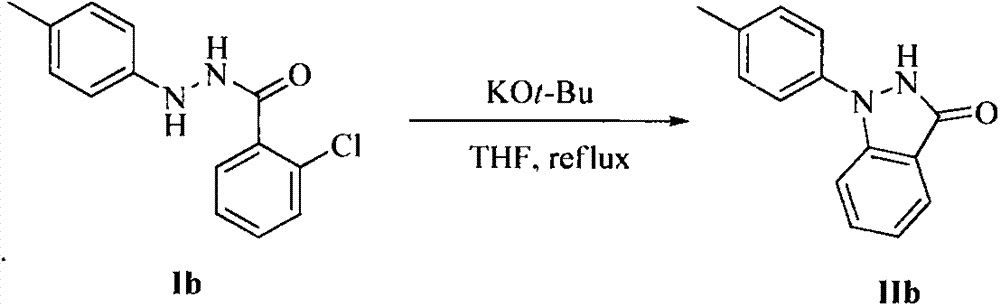
Method for synthesis of indazolone compound
A synthetic method and compound technology, applied in the direction of organic chemistry, can solve problems such as low yield, high requirements for reaction equipment, and many by-products, and achieve the effects of high reaction yield, high practical value, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
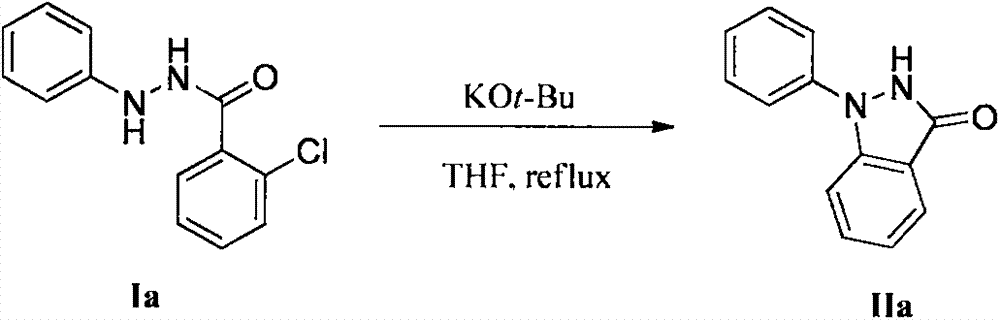
[0021] In a 250mL round bottom flask, add potassium tert-butoxide (3.0g, 27.0mmol), 2-chloro-N'-phenylbenzohydrazide Ia (2.2g, 9.0mmol), tetrahydrofuran (135mL), and Reflux for 2 hours. After the reaction, THF was removed under reduced pressure, water (50 mL) was added, extracted with ethyl acetate (50 mL×3), the combined extracts were washed with saturated brine, and the solvent was removed under reduced pressure to obtain a crude product. The crude product was purified by silica gel column chromatography using a mixed solvent of petroleum ether and ethyl acetate as eluent to obtain pure 1-phenylindazolone IIa (1.9 g, yield 99%). Melting point 165.0-166.9°C; 1 H NMR (400MHz, DMSO): δ11.24(s, 1H), 7.77(d, J=8.9Hz, 2H), 7.69(d, J=7.6Hz, 2H), 7.52(t, J=7.9Hz, 2H), 7.45(m, 1H), 7.26(t, J=7.4Hz, 1H), 7.16(t, J=7.7Hz, 1H); 13 C NMR (100MHz, DMSO): δ156.8, 140.7, 139.7, 123.0, 128.8, 125.2, 121.1, 121.0, 120.8, 115.3, 110.8; MS (ESI) C 13 h 11 N 2 O, [M+H] + : 211...
Embodiment 2
[0023] Using the same procedure as in Example 1, sodium tert-butoxide (2.6 g, 27.0 mmol) was used instead of potassium tert-butoxide to obtain 1-phenylindazolone IIa (0.61 g, yield 32%).
Embodiment 3
[0025] Using the same procedure as in Example 1, dimethylsulfoxide was used instead of N,N-dimethylformamide to obtain 1-phenylindazolone IIa (1.7 g, yield 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com