Tumor tissue in-vitro preservation liquid and preparation method thereof
A tumor tissue and preservation solution technology, applied in the biological field, can solve problems such as functional limitations, limited activity of tumor cells, and low success rate of tumor tissue transplantation, and achieve the effects of prolonging the storage time, increasing the success rate, and promoting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
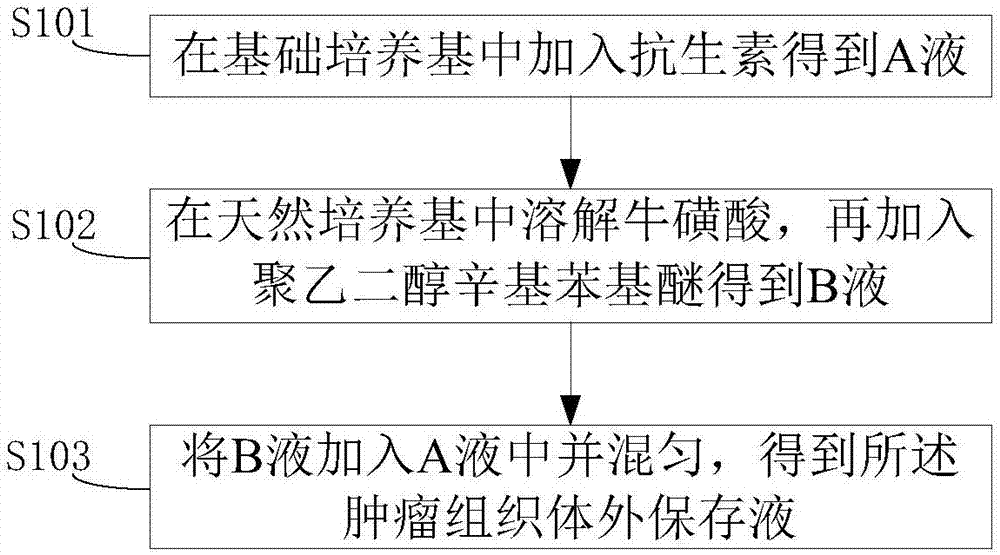
[0029] See figure 1 , figure 1 It is a schematic flow chart of an embodiment of the preparation method of the tumor tissue in vitro preservation solution of the present invention, and the method includes:
[0030] Step S101: Add antibiotics to the basic medium to obtain liquid A;
[0031] Step S102: Dissolve taurine in a natural medium, and then add polyethylene glycol octyl phenyl ether to obtain liquid B;
[0032] Step S103: adding liquid B to liquid A and mixing to obtain an in vitro preservation solution of tumor tissue.
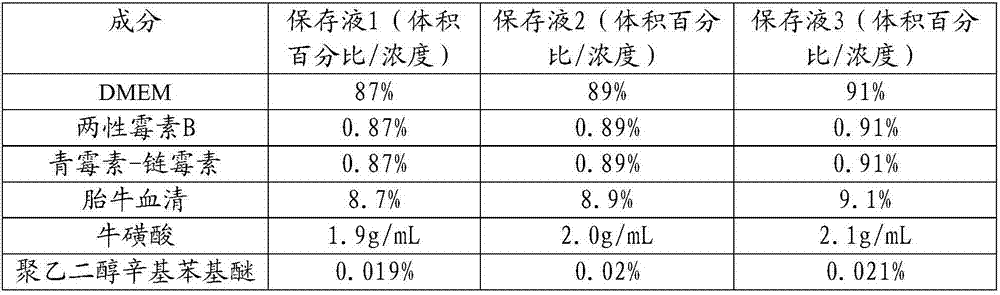
[0033] It should be noted that the various components used in the preparation of the tumor tissue in vitro preservation solution in this embodiment are the same as those in the first and second embodiments of the above-mentioned tumor tissue in vitro preservation solution of the present invention. For a detailed description of the relevant content, please Please refer to the above-mentioned embodiment, which will not be repeated here.
[0034] In an application s...
Embodiment 1
[0041] Divide the same volume of PBS buffer solution, cell culture solution and the tumor tissue in vitro preservation solution 1, 2, and 3 of the present invention into 5 groups as the preservation solution to preserve the tumor tissue. Among them, the preserved tumor tissue is of equal weight. The above 5 groups of tumor tissue transplantation experiments were completed within 2 hours. Each group was transplanted 5 6-week-old non-obese diabetes / severe combined immunodeficiency (Non ObeseDiabetes-Server Combined Immune-deficiency, NOD-SCID) mice subcutaneously. One month later, the tumorigenesis status of the tumor tissue was observed, and the success rate of colon cancer transplantation in the three groups was compared, as shown in Table 2. Compared with the other two groups, P <0.05.
[0042] It needs to be pointed out that, except for the above-mentioned differences, other conditions are the same.
[0043] Table 2 The success rate of colon cancer transplantation with each pre...
Embodiment 2
[0047] Divide the same volume of PBS buffer solution, cell culture solution and the tumor tissue in vitro preservation solution 1, 2, and 3 of the present invention into 5 groups as the preservation solution to preserve the tumor tissue. Among them, the preserved tumor tissue is of equal weight. The above 5 groups of tumor tissue transplantation experiments were completed within 6 hours. Each group was transplanted with 5 6-week-old NOD-SCID mice subcutaneously. One month later, the tumorigenesis status of tumor tissues was observed, and the success rate of colon cancer transplantation in the three groups Make a comparison, as shown in Table 3. Compared with the other two groups, P <0.05.
[0048] It needs to be pointed out that, except for the above-mentioned differences, other conditions are the same.
[0049] Table 3 The success rate of colon cancer transplantation with each preservation solution
[0050]
PBS buffer
Cell culture medium
Preservation solution 1
Preservatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com