Method for quantitatively detecting glial fibrillary acidic protein (GFAP) in blood serum
A kind of neuroglial, quantitative detection technology, applied in the fields of nanomaterials, fluorescence immunology technology and biological analysis detection, to achieve the effect of reducing interference and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of protein A / G agarose purified resin-linked antibody (PA / G-Ab1), the steps are as follows:
[0036] Add 10 μL of protein A / G agarose purification resin to a centrifuge tube, add 0.5 mL of binding buffer to resuspend the resin, centrifuge at 3000 rpm for 3 minutes, discard the supernatant, and repeat twice; add mouse anti-human GFAP to the balanced PA / G Monoclonal IgG 45μL (20μg / mL), 100rpm, shake and mix at 25°C for 1h; after the reaction, add 1% BSA and react for 1h to block the non-specific sites on the antibody; the reaction product PA / G-Ab1 was mixed with binding buffer Wash with liquid, centrifuge at 3000rpm for 3min, discard the supernatant, repeat twice to remove unreacted Ab1 and impurities, and store the product at 4°C until use.
Embodiment 2
[0037] The preparation of embodiment 2. carbon dots (CDs), the steps are as follows:
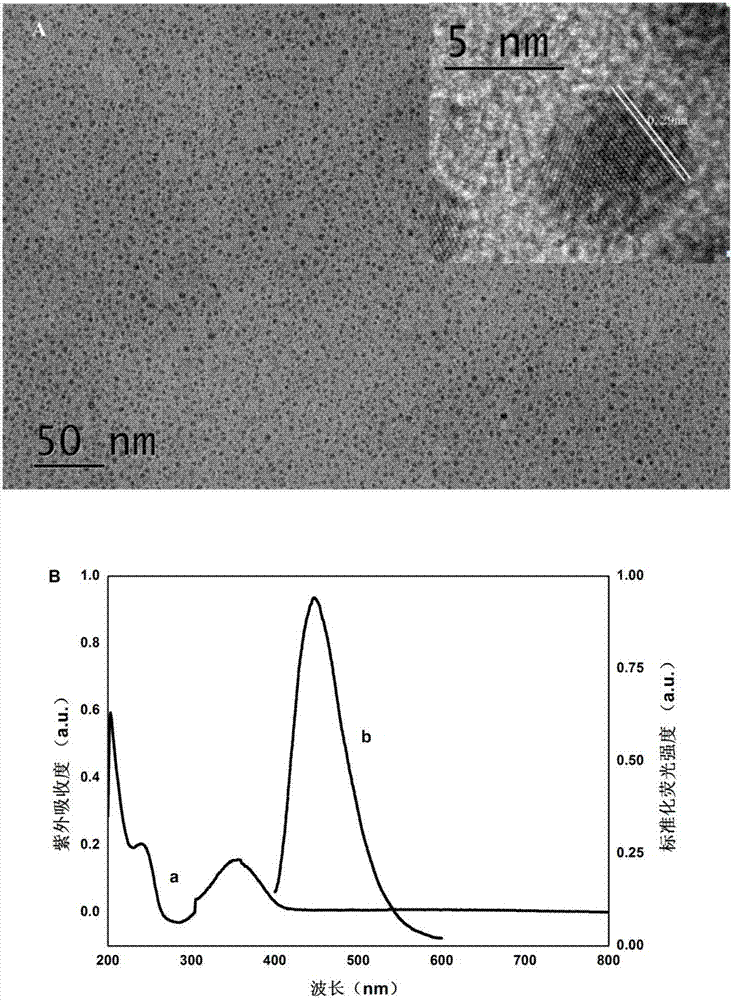
[0038] Take 1.2g of citric acid monohydrate, 1.2mL of diethylenetriamine and 20mL of ultrapure water in a 30mL autoclave, fully ultrasonicate for 15min to dissolve, and react at 200°C for 6h; take out the autoclave and place it at room temperature to obtain a brown solution. That is the CDs solution; in order to purify CDs, add phosphoric acid to adjust the pH to about 7, dialyze overnight with a 1000Da dialysis bag to remove unreacted impurities; collect the dialyzed CDs solution, suspend under reduced pressure, concentrate and dry at low temperature; the obtained CDs are pale yellow powders. Its transmission electron microscope image ( figure 1 A) UV absorption and fluorescence emission diagrams ( figure 1 B).
Embodiment 3
[0039] The preparation of embodiment 3 fluorescently labeled detection antibodies (CDs-Ab2), the steps are as follows:
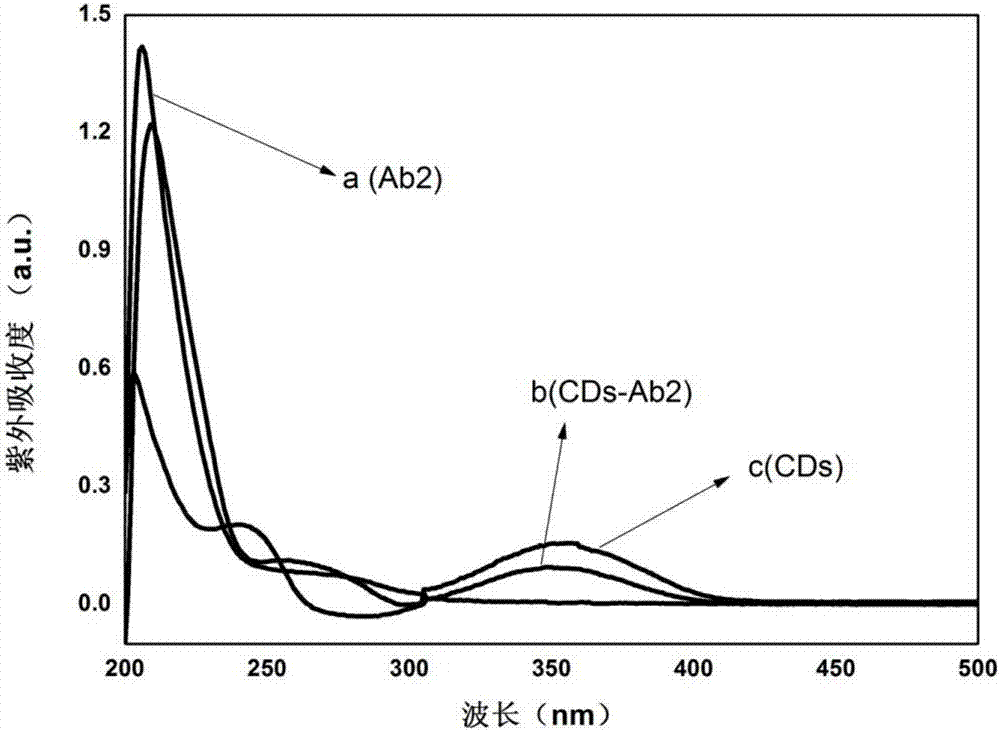
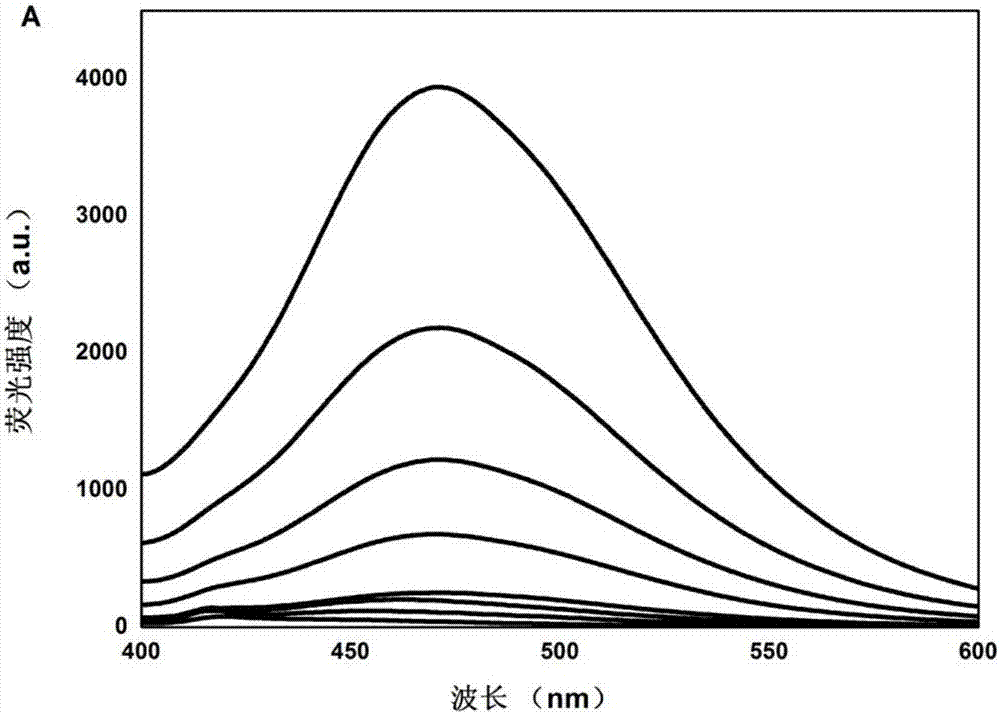
[0040] Dissolve CDs in 10mM PBS to make a 200μg / mL solution; add 30μL each of EDC and NHS (200mg / mL) to 150μL CDs, add NaOH to adjust the pH of the solution to about 7.5, and ultrasonically react for 30min; add rabbit anti-human GFAP Clone IgG, 200rpm, shaking reaction at 25°C for 2.5h; the reaction system was blocked with 1% BSA to block the non-specific site of the antibody, and reacted at 25°C for 1h; Fluorescence; the resulting product CDs-Ab2 (b) and Ab2 (a), CDs (c) were measured ultraviolet, the results are as follows figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com