Preparation method of stable nanometer suspensions of mederate hydrophobic medicine
A nanosuspension, hydrophobic drug technology, applied in pharmaceutical formulations, medical preparations of inactive ingredients, capsule delivery, etc., can solve the problems of high particle load and high stability of particle size that cannot be satisfied at the same time. , to achieve the effect of long dimensional stability, small average particle size and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 30 mL end-aminated mPEG- b -Suspension of PLGA-stabilized curcumin nanoparticles (containing 3 mgmPEG- b -PLGA and 3 mg curcumin):
[0026] At room temperature, take 3 mg of mPEG- b -PLGA, 3 mg curcumin (LogP is 3) and 3 mL tetrahydrofuran were thoroughly mixed to obtain a mixed solution, which was inhaled into a syringe.
[0027] Draw 3 mL of pH 9 water into another syringe of the same size.
[0028] Connect the two syringes and the two inlets of the mixer respectively, inject the two liquids into the mixer at a speed of 0.1 m / s at the same time, and the volume of the closed cavity of the mixer is about 30uL. The two-phase liquid is fully turbulently mixed in the mixer cavity, and the resulting emulsion flows out from the outlet of the mixer. Mixing takes about 3 seconds in total and the suspension is collected in a container.
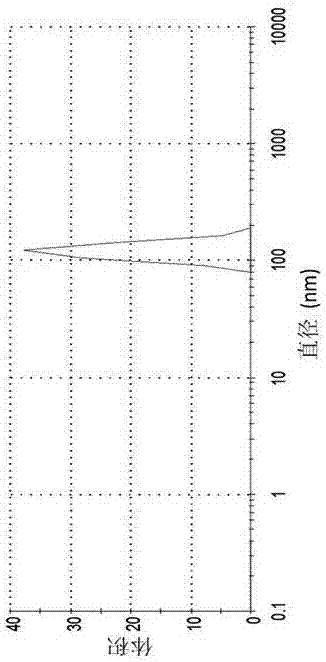
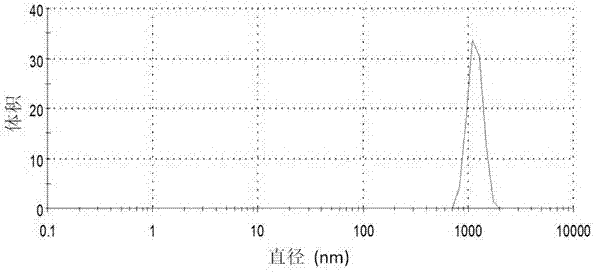
[0029] Be that the water dilution suspension of 9 is to 5 times with pH, and measure the particle size and di...
Embodiment 2
[0031] Example 2: Preparation of 30 mL hydroxyl-terminated mPEG- b - PLGA-stabilized suspension of curcumin nanoparticles (containing 3 mgmPEG- b -PLGA and 3 mg curcumin):
[0032] At room temperature, take 3 mg of hydroxyl-terminated mPEG- b -PLGA, 3 mg curcumin and 3 mL tetrahydrofuran were mixed thoroughly and inhaled into a syringe.
[0033] Draw 3 mL of pH 9 water into another syringe of the same size.
[0034] Connect the two syringes and the two inlets of the mixer with screw-on heads respectively, and inject the two liquids into the mixer at the same speed at the same time. The two-phase liquid is fully turbulently mixed in the mixer cavity, and the resulting emulsion flows out from the outlet of the mixer. Mixing took about 3 seconds in total. Collect the suspension in a container.
[0035] Be that the water dilution suspension of 9 is to 5 times with pH, and measure the particle size and distribution of suspension with dynamic light scattering method, the res...
Embodiment 3
[0037] Example 3: Comparison of 30 mL end-aminated mPEG- b - Temporal stability of PLGA-stabilized curcumin nanoparticle suspension (containing 3 mg terminally aminated mPEG- b -PLGA, 3 mg curcumin or 3 mg aminated mPEG- b -PLGA, 0.6 mg curcumin)
[0038] At room temperature, take 3 mg of mPEG- b - PLGA, 3 mg or 0.6 mg curcumin and 3 mL tetrahydrofuran are thoroughly mixed and inhaled into a syringe.
[0039] Draw 3 mL of water of different pH values into another syringe of the same size.
[0040] Connect the two syringes to the two inlets of the mixer, and simultaneously inject the two liquids into the mixer at a speed of 0.1 m / s. The two-phase liquid is fully turbulently mixed in the mixer cavity, and the resulting suspension flows out from the mixer outlet. Mixing took about 3 seconds in total. Collect the suspension in a container.
[0041] Dilute the suspension to 5 times with water of the same pH, and use the dynamic light scattering method to analyze the partic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com