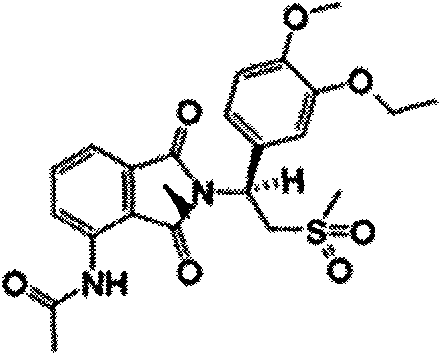
Apremilast oral solid preparation and preparation method thereof
A technology of solid preparations and fillers, applied in anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc., which can solve problems such as insufficient oral release, low dissolution of apremilast, and complicated operation processes, and achieve stable and reliable quality and reduce The effect of simple production cost and equipment operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0025] Embodiments 1-6 Apremilast oral solid preparation and preparation method thereof
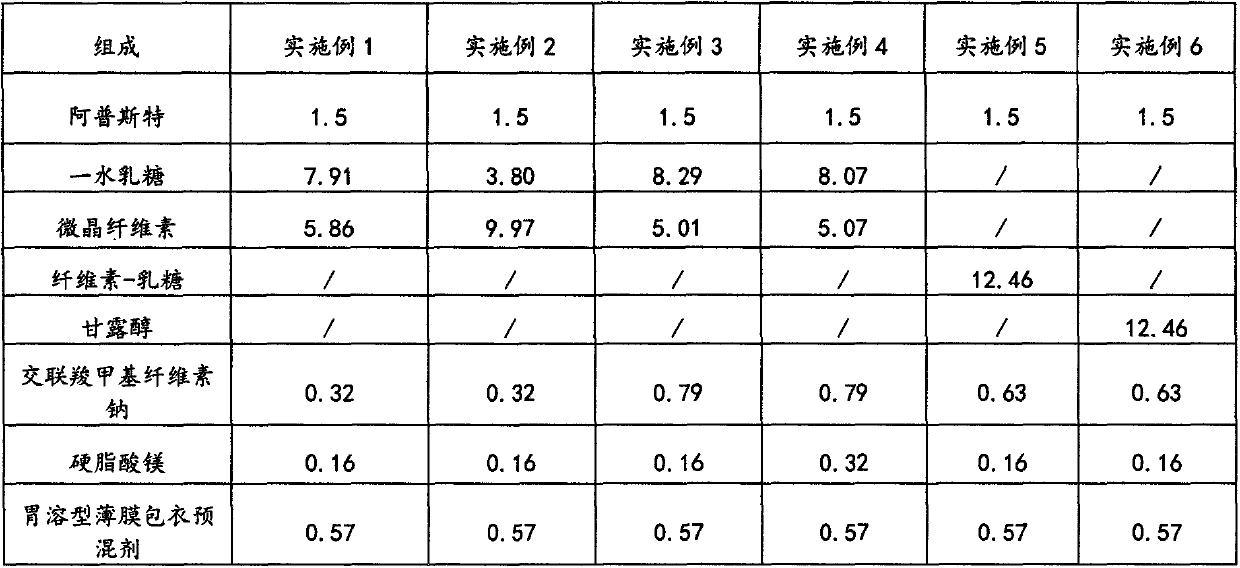
[0026] The formulations of the oral solid preparations of apremilast in Examples 1-6 are as described in Table 1.
[0027] The formula (unit: g) of the apremilast oral solid preparation of table 1 embodiment 1~6
[0028]
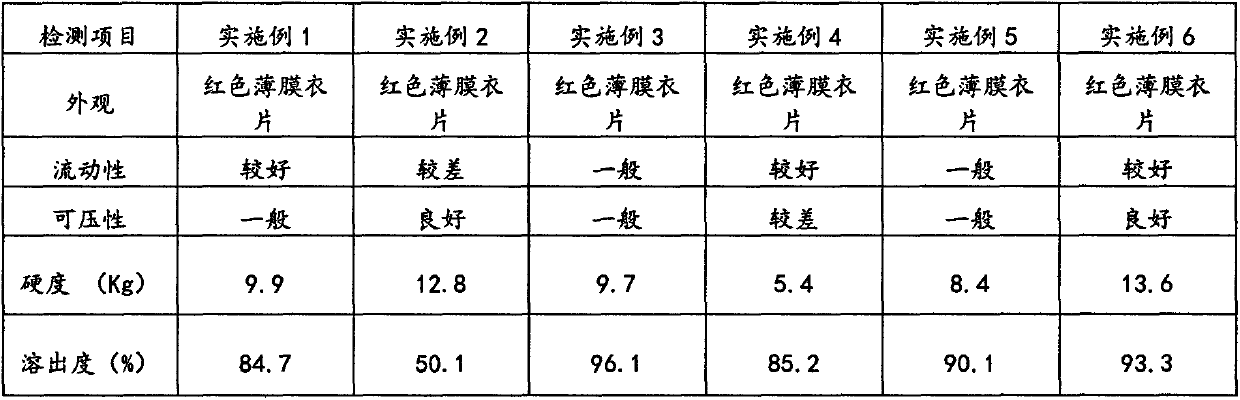
[0029] The preparation method of the Apremilast tablet of above embodiment 1~6 is as follows:
[0030] (1) passing the active ingredient of the drug through a 120-mesh sieve for subsequent use; fillers and disintegrants through a 120-mesh sieve for subsequent use;
[0031] (2) Weighing and premixing: mix the prescribed amount of API with fillers and disintegrants in a mixer;
[0032] (3) add lubricant to above-mentioned mixed powder, tabletting;
[0033] (4) will The powder is formulated into a solution and coated, and the coating temperature is controlled at 38°C to 42°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com