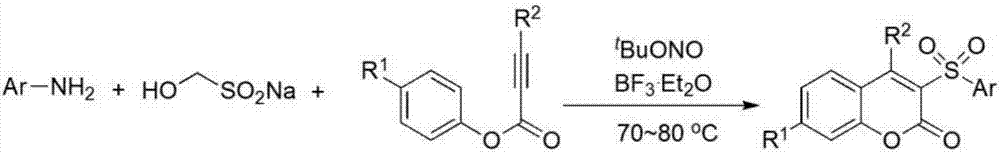
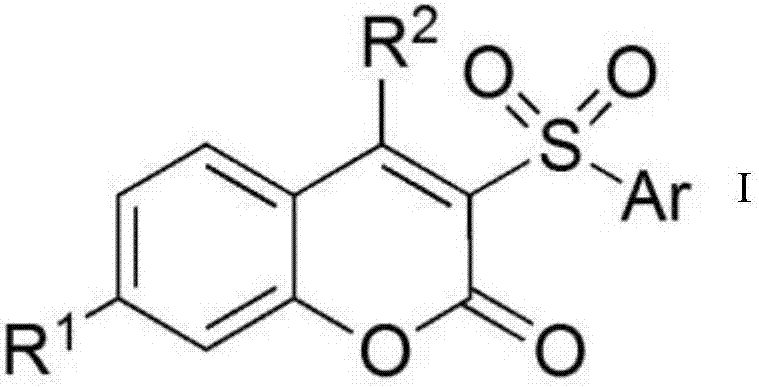
3-Sulfonylcoumarin and preparation method thereof
A technology for coumarin and coumarin derivatives, applied in the field of coumarin, can solve problems such as unfavorable operation and use, odor, etc., and achieve the effects of avoiding the use of sulfonyl chloride, simple and easy-to-obtain raw materials, and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A 3-sulfone coumarin, the structural formula is:
[0021] The preparation method is: add 1.5 equivalents of aromatic amine, 1.5 mL of 1,2-dichloroethane, 1.8 equivalents of tBuONO, 1.5 equivalents of boron trifluoride ether solution into the reaction tube, and under the protection of inert gas nitrogen or argon, Stir at 0°C for 10 minutes; then add 0.2mmol of phenyl phenylpropiolate, 1.6 equivalents of white block and 2.0mL of 1,2-dichloroethane, stir at 70~80°C for 5.0-6.0 hours, until TLC detection is complete reaction. After the reaction liquid was washed with water, extracted with ethyl acetate, the extract was concentrated and separated by column chromatography to obtain the corresponding 3-sulfone coumarin Ia with a reaction yield of 83%.
[0022] The structure of the compound 1 H NMR, 13 C NMR, HRMS and other methods are characterized and confirmed: 1 H NMR(400MHz, CDCl 3 )δ7.95(d,J=8.9Hz,2H),7.56-7.58(m,3H),7.32-7.34(m,2H),7.14(s,1H),6.95-7.00(m,3H),6.88 (d,J=8.3,...
Embodiment 2
[0024] A 3-sulfone coumarin with the structural formula
[0025] The preparation method is as follows: add aromatic amine (1.5 equivalents), 1,2-dichloroethane (1.5mL), tBuONO (1.8 equivalents), boron trifluoride ether solution (1.5 equivalents) in order in the reaction tube, Under the protection of nitrogen or argon, stir at 0°C for 10 minutes; then add phenyl phenylpropiolate (0.2mmol), white cubes (1.6 equivalent) and 1,2-dichloroethane (2.0mL), at 70 Stir at ~80°C for 5.0-6.0 hours until TLC detects complete reaction. After the reaction liquid was washed with water, extracted with ethyl acetate, the extract was concentrated and separated by column chromatography to obtain the corresponding 3-sulfone coumarin Ib, with a reaction yield of 84.5%.
[0026] The structure of the compound 1 H NMR, 13 C NMR, HRMS and other methods are characterized and confirmed: 1 H NMR(400MHz, CDCl 3 )δ7.94(d,J=8.6Hz,2H),7.58-7.89(m,3H),7.47(d,J=8.6Hz,2H),7.32-7.34(m,2H),7.16(s,1H) ), 7.02 (d, J =...
Embodiment 3
[0028] A 3-sulfone coumarin with the structural formula
[0029] The preparation method is as follows: add aromatic amine (1.5 equivalents), 1,2-dichloroethane (1.5mL), tBuONO (1.8 equivalents), boron trifluoride ether solution (1.5 equivalents) in order in the reaction tube, Under the protection of nitrogen or argon, stir at 0°C for 10 minutes; then add phenyl phenylpropiolate (0.2mmol), white cubes (1.6 equivalent) and 1,2-dichloroethane (2.0mL), at 70 Stir at ~80°C for 5.0-6.0 hours until TLC detects complete reaction. After the reaction liquid was washed with water, extracted with ethyl acetate, the extract was concentrated and separated by column chromatography to obtain the corresponding 3-sulfone coumarin Ic with a reaction yield of 85%.
[0030] The structure of the compound 1 H NMR, 13 C NMR, HRMS and other methods are characterized and confirmed: 1 H NMR(400MHz, CDCl 3 )δ7.87(d,J=8.7Hz,2H),7.64(d,J=8.7Hz,2H),7.58-7.59(m,3H),7.32-7.34(m,2H),7.17(s,1H) ), 7.02 (d, J = 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com