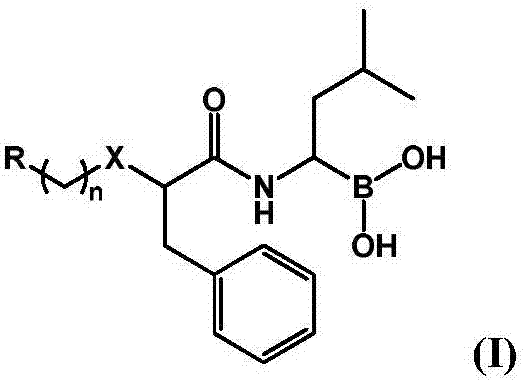
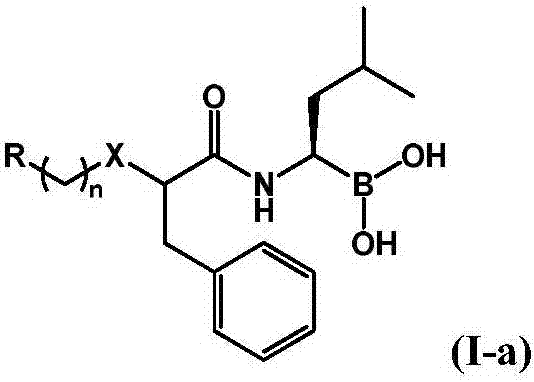
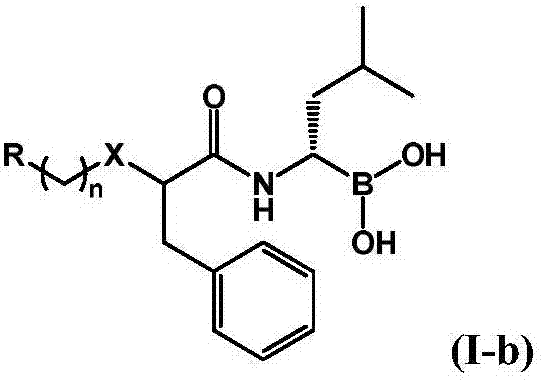
Boric acid compound used as 20S proteasome inhibitor, and preparation method thereof
A proteasome inhibitor and proteasome technology, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve problems such as unclearness and inconvenience to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: Preparation of dichloromethylenelithium (compound III-2)
[0090] LiCHCl 2
[0091] (III-2)
[0092] Under the condition of nitrogen protection, add anhydrous dichloromethane (4.6mL, 72mmol) to 200mL of anhydrous tetrahydrofuran, the temperature dropped to -110 ℃, dropwise added 1.6M n-butyllithium n-hexane solution (38mL, 60mmol ), after the dropwise addition, continued to stir at -110°C for 1 hour; the reaction solution was directly used in the next reaction without purification.
Embodiment 2
[0093] Embodiment 2: Preparation of dimethyl dichloromethylene borate (compound III-3)
[0094]
[0095] Under the condition of nitrogen protection, the temperature was continued to be controlled at -110°C, trimethyl borate (8mL, 72mmol) was added to the compound III-2 solution prepared in Example 1, and stirring was continued at -110°C for 1 hour, and then Add 12mL of 5N HCl solution, the reaction is slowly raised to room temperature, transfer the reaction solution into a separatory funnel, separate the organic phase, extract the aqueous phase with ether (3×10mL), combine the organic phases, add anhydrous sodium sulfate to dry, dry After the completion of the filtration, the solvent was removed by a rotary evaporator to obtain 8.7 g of a white solid with a yield of 92%. The reaction product was directly used in the next reaction without purification.
Embodiment 3
[0096] Embodiment 3: Preparation (+)-α-pinanediol (compound III-4-a)
[0097]
[0098] Under nitrogen protection, add Me to 100 mL tert-butanol 3 NO·2H 2 O (11g, 102mmol) in aqueous solution was added (+)-α-pinene (15mL, 97mmol), pyridine (7mL) and osmium tetroxide (51mg, 0.2mmol) successively under stirring, and then heated to reflux, and after 24 hours TLC detection shows that the reaction is complete, the reaction is down to room temperature, and NaHSO is added 3 (1.2g, 12mmol) was stirred for 1 hour, the reaction solution was transferred to a separatory funnel, the organic phase was separated, the aqueous phase was extracted with ether (3×20mL), the organic phases were combined, dried by adding anhydrous sodium sulfate, and after drying Filtrate, remove the solvent with a rotary evaporator, and separate by column chromatography (petroleum ether: ethyl acetate = 30:1) to obtain 15 g of a white solid with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com