Traditional Chinese medicine extract composition for treating cervical cancer
The technology of extract and composition is applied in the field of natural traditional Chinese medicine extract composition to prepare and treat cervical cancer drug development field, which can solve the problems of large dosage of single Chinese herbal medicine, difficult to control toxic and side effects, limited anti-cancer effect, etc. The process is simple and easy to operate, easy to put into production, and obtain convenient results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Observation and determination of drug toxicity test of Chinese medicine extract composition.
[0020] Experimental materials: (1) Experimental cells: human cervical cancer HeLa cell line: purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences, subcultured by conventional methods; (2) Experimental drugs: dried fruit of the Chinese medicine Forsythia, produced in Shanxi; Chinese medicine licorice root, produced in Inner Mongolia , were purchased from the Outpatient Department of Guoyitang, Heilongjiang Association of Traditional Chinese Medicine, and passed the identification of the Chinese Medicine Department of Heilongjiang Food and Drug Inspection and Testing Institute.
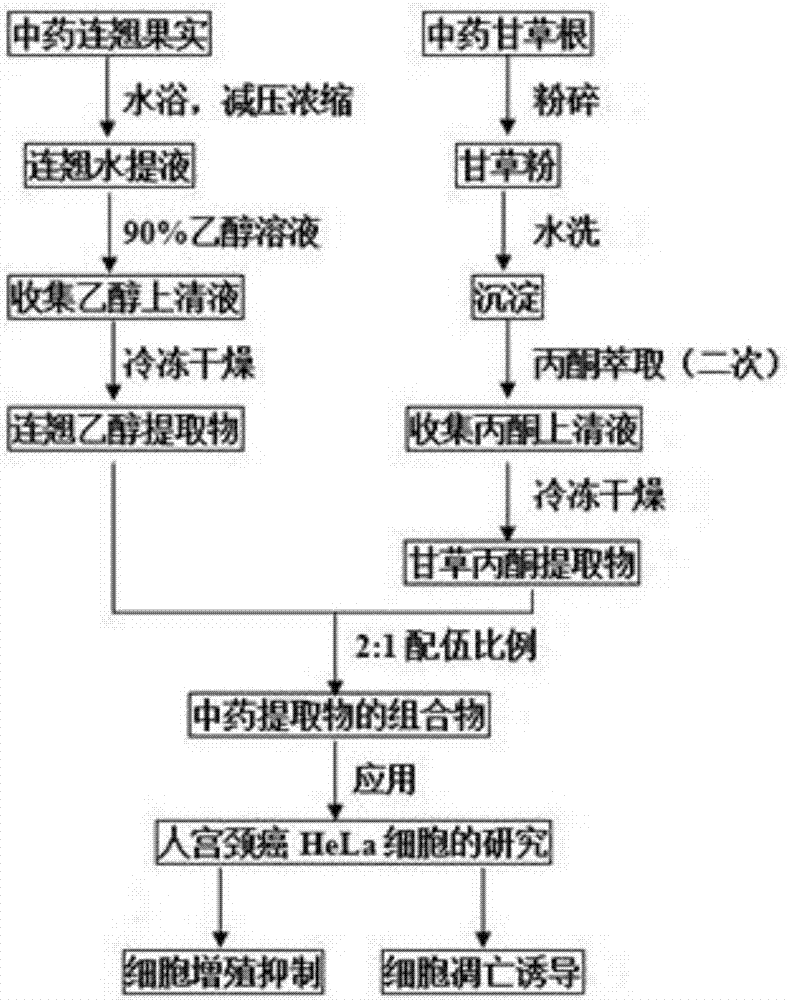
[0021] Experimental method: (1) Extraction of the ethanol extract of the traditional Chinese medicine Forsythia was purified according to conventional methods: the specific steps were to take the dried fruit of Forsythia, wash and remove dust, soak in distilled water overni...
Embodiment 2
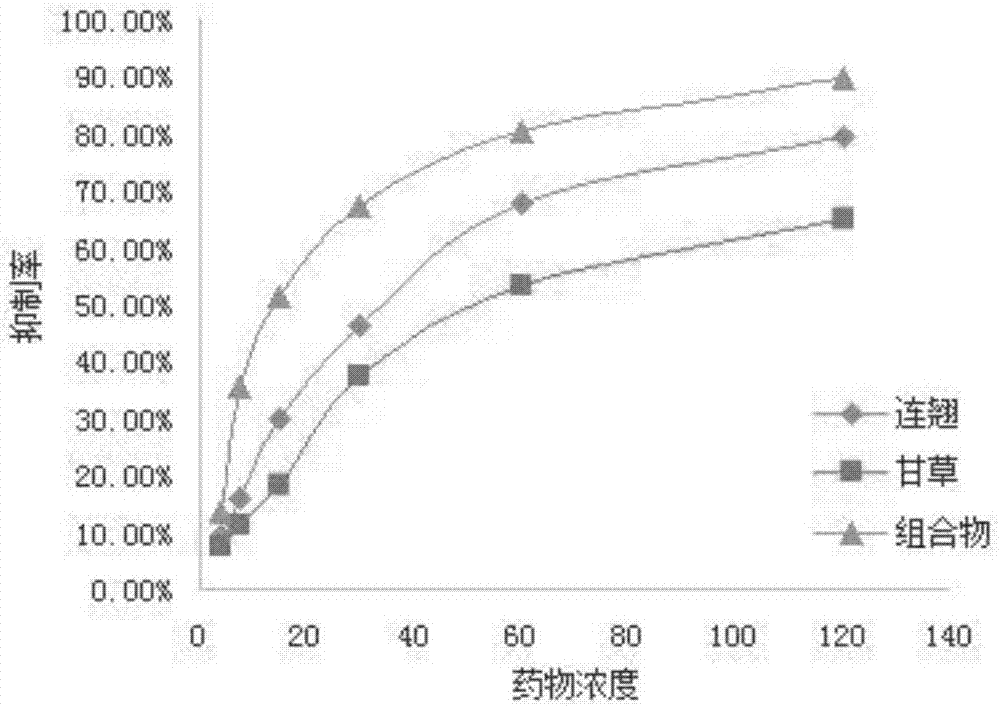
[0027] Example 2: MTT colorimetric detection of the Chinese medicine extract composition on HeLa cell proliferation inhibition rate and cytotoxicity (IC50) determination.
[0028] 1. Experimental materials: the same as in Example 1.
[0029]2. Experimental method: HeLa cells in good logarithmic growth phase were digested into a cell suspension, and the cell density was adjusted with cell culture medium to make it reach 1.0×105 cells / mL. The cell suspension was added to a 96-well plate at 100 μL / well, and incubated in a CO2 incubator for 24 hours. The drug ratio is 2:1, the final drug concentration is 120 μg / mL, 60 μg / mL, 30 μg / mL, 15 μg / mL, 7.5 μg / mL, 3.75 μg / mL, 100 μL per well. And set up forsythia ethanol extract group, licorice acetone extract group, cisplatin (DDP) drug control group, vehicle negative control group (the cell growth will not be affected when the alcohol concentration is lower than 0.5%) and blank control group (only culture solution added) , without cell...
Embodiment 3
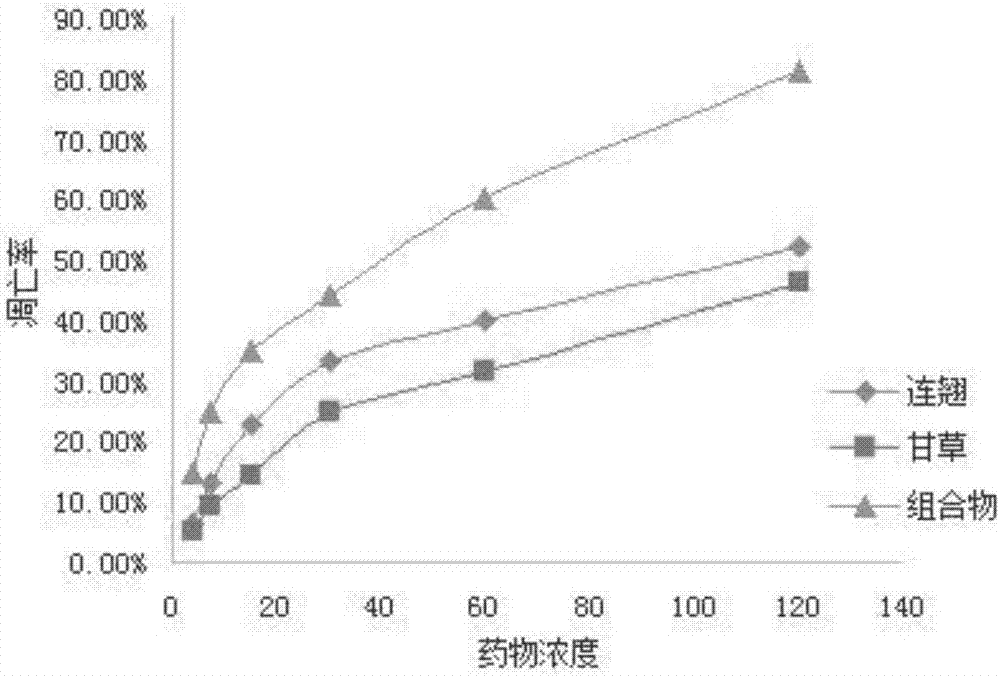
[0033] Example 3: The application of flow cytometer AnnexinV-PI double staining method to detect the apoptosis of human cervical cancer HeLa cells induced by the Chinese medicine extract composition.
[0034] Experimental material: with embodiment 1.
[0035] Experimental method: HeLa cells in good logarithmic growth phase were inoculated into 50mL culture flasks, incubated in a 37°C, 5% CO2 incubator, and the number of cells was adjusted to 1×106 cells / mL. Dilute the final concentration of the Chinese medicine extract composition (2:1) to 120 μg / mL, 60 μg / mL, 30 μg / mL, 15 μg / mL, 7.5 μg / mL, and 3.75 μg / mL in a 50 mL culture bottle, and set up a continuous Alice ethanol extract control group, licorice acetone extract control group, vehicle negative control group. Incubate for 24 hours in a 37°C, 5% CO2 incubator. Centrifuge at 4°C and 1000r / min for 10 min, discard the supernatant and collect the cells, repeat the above conditions and wash twice with PBS. The cells were resus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com