Folic acid-chitosan-Cy7 polymer with tumor targeting ability and preparation method of polymer
A technology of tumor targeting and chitosan derivatives, which is applied in the field of biomedicine and can solve problems such as poor solubility of chitosan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Polymer CF7:
[0044] Step a: Weigh 800 mg of chitosan and dissolve in 60 mL of anhydrous DMF, then add 1.6 g of phthalic anhydride, under nitrogen protection, stir and heat in an oil bath at 120°C. When the reaction solution became clear, the reaction was terminated. The reaction solution was poured into an appropriate amount of ice water, and a white precipitate was precipitated. After suction filtration, the solid was washed three times with ether and acetone respectively to remove excess phthalic anhydride, and dried to obtain product 2.
[0045] Step b: Weigh 100 mg of product 2, add 10 mL of N-methylpyrrolidone (NMP), heat and stir to dissolve. When the solution was cooled and placed in ice water, 616 mg N-bromosuccinimide (NBS) and 902 mg triphenylphosphine (TPP) were added. React at 80°C for two hours under nitrogen protection. After the reaction was over, the mixture was poured into 100 mL of ethanol, and a solid was precipitated. The product ...
Embodiment 2
[0049] Synthesis of Chitosan-Cy7 Polymer (C7):
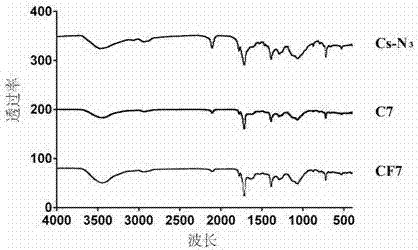
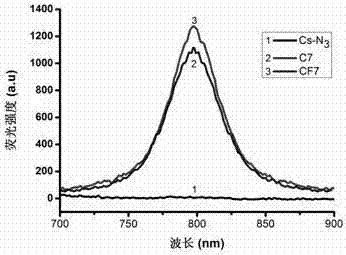
[0050] Weigh 10 mg of the product 4 of Example 1, dissolve it in 5 mL of dimethyl sulfoxide, and add 10 mg of ALK-Cy7. The flask was sealed with a rubber stopper and protected with nitrogen after evacuation. First add 2.5 mg copper sulfate pentahydrate (dissolved in 100 μL secondary water) dropwise to the flask with a 1 mL syringe, and then add 2 mg sodium ascorbate (dissolved in 100 μL secondary water) dropwise. The reactants were reacted at 50 °C for 72 h in the dark. After the reaction, the reaction solution was dialyzed for 72 hours with a 14000 dialysis bag. After dialysis, the product was lyophilized. According to infrared spectrogram analysis, the 6-position azido group in product 4 reacted with the alkynyl group in ALK-Cy7 to form a triazole ring. Such as figure 1 As shown, chitosan-Cy7 polymer (C7) at 2100 cm -1 There is no infrared absorption peak, indicating that the azido group has successfully reacted with th...
Embodiment 3
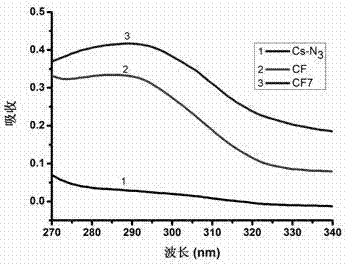
[0052] Synthesis of chitosan-FA polymer (CF):
[0053] Weigh 10 mg of the product 4 of Example 1, dissolve it in 5 mL dimethyl sulfoxide, and add 10 mg ALK-FA. The flask was sealed with a rubber stopper and protected with nitrogen after evacuation. First add 2.5 mg copper sulfate pentahydrate (dissolved in 100 μL secondary water) dropwise to the flask with a 1 mL syringe, and then add 2 mg sodium ascorbate (dissolved in 100 μL secondary water) dropwise. The reactants were reacted at 50 °C for 72 h in the dark. After the reaction, the reaction solution was dialyzed for 72 hours with a 14000 dialysis bag. After dialysis, the product was lyophilized. According to infrared spectrogram analysis, the azido group at position 6 in product 4 reacted with the alkynyl group in ALK-FA to form a triazole ring. Such as figure 1 As shown, chitosan-FA polymer (CF) at 2100 cm -1 There is no infrared absorption peak, indicating that the azido group has successfully reacted with the alkyn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com