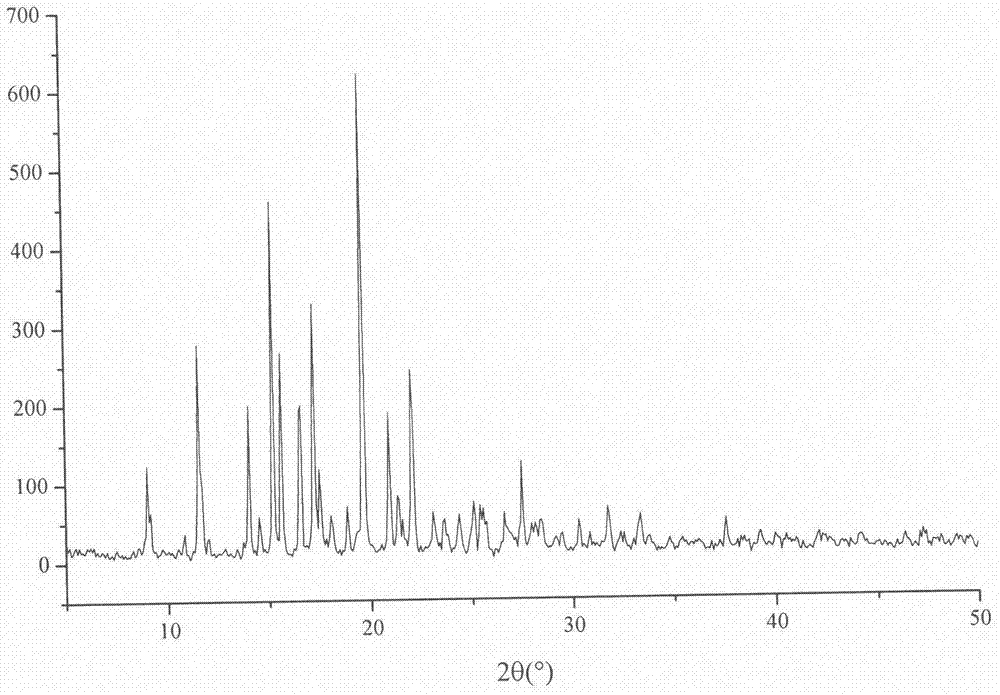
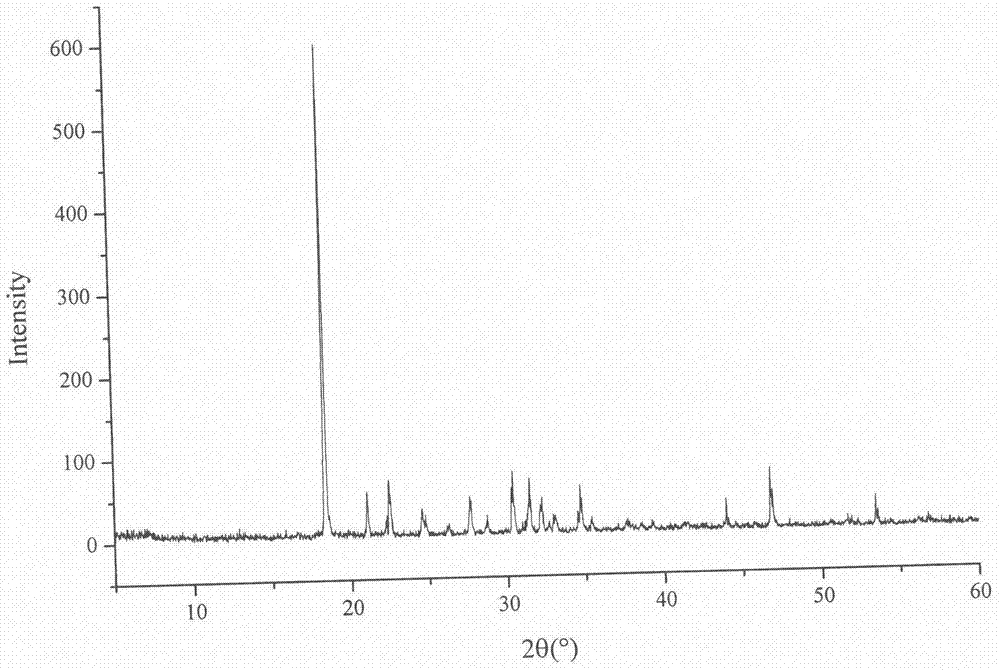
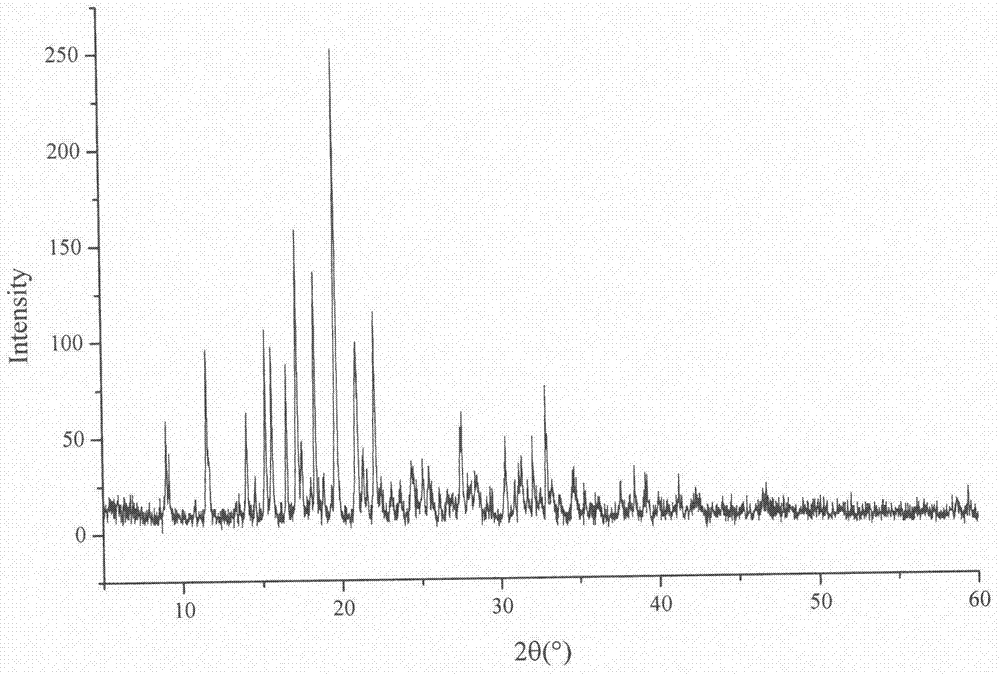
Lurasidone hydrochloride-amino acid coamorphous material
A technology of lurasidone hydrochloride and amorphous substance, applied in the field of medicine, can solve the problems of low dissolution rate, poor water solubility, low oral bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of lurasidone hydrochloride L-cysteine hydrochloride co-amorphous
[0065] Add 0.50g of lurasidone hydrochloride and 0.15g of L-cysteine hydrochloride into 250mL of ethanol, and ultrasonically dissolve at room temperature to obtain a clear solution. Rotate the solvent under reduced pressure at 40°C to dry the clear solution under vacuum at 25°C. After 24 hours, 0.38 g of white powder was obtained.
Embodiment 2
[0066] Example 2: Preparation of lurasidone hydrochloride L-cysteine hydrochloride co-amorphous
[0067] Add 0.50g of lurasidone hydrochloride and 0.15g of L-cysteine hydrochloride into 50mL of methanol, and ultrasonically dissolve at room temperature to obtain a clear solution. Rotate the solvent under reduced pressure at 50°C to dry the clear solution under vacuum at 25°C. After 24 hours, 0.53 g of white powder was obtained.
Embodiment 3
[0068] Example 3: Preparation of lurasidone hydrochloride L-cysteine hydrochloride co-amorphous
[0069] Add 0.50 g of lurasidone hydrochloride and 0.15 g of L-cysteine hydrochloride into 60 mL of methanol-ethanol (50:50, v / v) mixed solvent, and ultrasonically dissolve at room temperature to obtain a clear solution. The solvent was rotary evaporated under reduced pressure at 45°C, and dried in vacuum at 25°C for 24 hours to obtain 0.51 g of white powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com