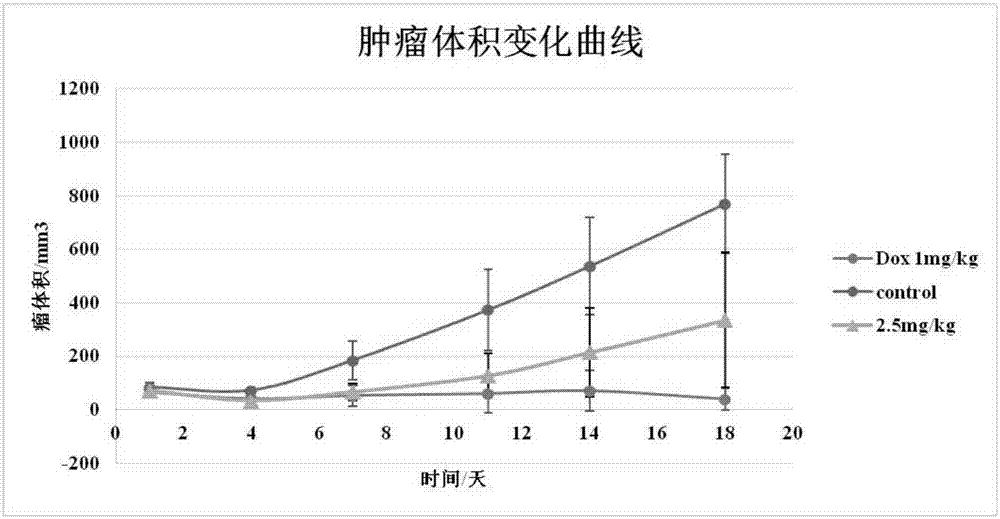
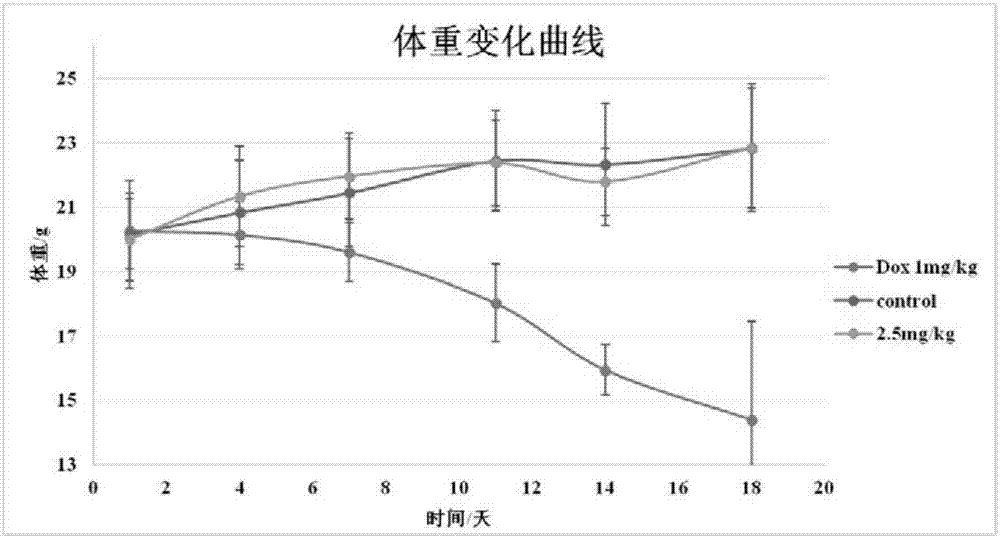
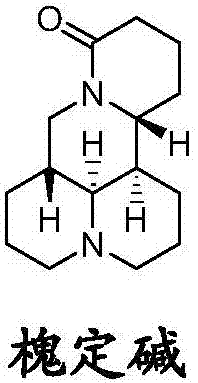
Sophoridine amine derivative, preparation method thereof and application
A compound and solvate technology, applied in the field of medicine, can solve problems such as less research on structure-activity relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1 Synthesis of 12-N-p-chlorobenzyl sophoridin aldehyde (Q08)
[0108]
[0109] Step 1: Sophoridine (4.96g, 20mmol) was uniformly dispersed in 50mL 6N HCl aqueous solution, and refluxed for 4 hours. After TLC detected that the raw material disappeared, the reaction was stopped, and the solvent was evaporated to dryness under reduced pressure to obtain a yellow oil, and 50mL of anhydrous Methanol was stirred at room temperature for two hours, TLC detected that the reaction was complete, and the solvent was evaporated to dryness under reduced pressure to obtain a white solid, which was separated and purified by silica gel column chromatography using dichloromethane / methanol as the mobile phase to obtain a white solid Z0A.
[0110] Step 2: Suspend the above white solid ZOA (2.81g, 10.0mmol) in 50mL 1,2-dichloroethane, add triethylamine (2.8mL, 20.0mmol), stir until dissolved, add p-chlorobenzaldehyde ( 2.1g, 15.0mmol), after reflux for 2 hours, slowly add sodi...
Embodiment 2
[0113] Example 2 Synthesis of 12-N-p-chlorobenzyl-4'-(3,4,5-trimethoxyphenyl)sophoridine (A08A)
[0114]
[0115] Dissolve Q08 (1.1g, 3.0mmol) in 50mL of anhydrous methanol, add 3,4,5-trimethoxyaniline (0.8g, 4.5mmol), and after refluxing for 4 hours, slowly add sodium cyanoborohydride (0.9 g, 4.5mmol), continue to reflux for 4 hours, after cooling to room temperature, evaporate the reaction system to dryness under reduced pressure, add 50mL of dichloromethane, wash with 30mL water and 30mL saturated ammonium chloride solution successively, and evaporate the organic phase to dryness under reduced pressure , using dichloromethane / methanol as the mobile phase, separated and purified by silica gel column chromatography to obtain a white solid (1.1 g, 67%). Melting point: 77-79°C; 1 H NMR (500MHz, DMSO-d 6 )δ7.43–7.25(m,4H),5.86(s,2H),3.70(s,6H),3.62–3.54(m,1H),3.53(s,3H),3.50–3.44(m,1H) ,3.09–2.63(m,6H),2.50–2.37(m,3H),2.30–2.12(m,1H),2.13–1.84(m,3H),1.81–1.60(m,3H),1.61–1...
Embodiment 3
[0116] Example 3 Synthesis of 12-N-p-chlorobenzyl-4'-(N-morpholinyl)sophoridine (A08B)
[0117]
[0118] Dissolve Q08 (1.1g, 3.0mmol) in 50mL of anhydrous methanol, add 4-aminomorpholine (0.43mL, 4.5mmol), reflux for 4 hours, cool to room temperature, evaporate the reaction system to dryness under reduced pressure, add 50mL di After methyl chloride, wash with 30mL water and 30mL saturated ammonium chloride solution successively, evaporate the organic phase to dryness under reduced pressure, use dichloromethane / methanol as the mobile phase, and separate and purify by silica gel column chromatography to obtain a white solid (1.0g, 74 %). Melting point: 59-61°C; 1 H NMR (500MHz, DMSO-d 6 )δ7.38–7.30(m,4H),6.93(t,J=5.2Hz,1H),3.73–3.65(m,4H),3.61–3.44(m,2H),3.01–2.89(m,1H) ,2.88–2.75(m,6H),2.71–2.60(m,1H),2.50–2.35(m,2H),2.25–1.87(m,6H),1.82–1.62(m,3H),1.58–1.37( m,5H),1.31–1.21(m,2H),1.17–1.10(m,1H),1.01(dd,J=20.5,11.7Hz,1H); 13 C NMR (125MHz, DMSO-d 6 )δ140.4, 139.7, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com