Establishment method of programmed necrosis inducing system
A technology of programmed necrosis and establishment method, applied in the field of establishment of programmed necrosis induction system, can solve problems such as poor effect, and achieve the effect of high efficiency, rapid induction of cell necrosis and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be described in detail below in combination with specific embodiments.
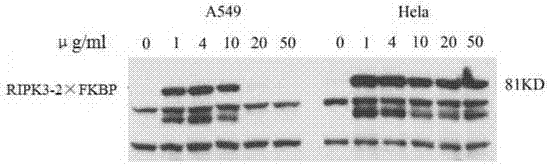
[0022] In the present invention, through the puromycin screening of the lentiviral packaging system, we have constructed stable cell lines of A549 and Hela cells that can induce the expression of RIPK3-2×FKBP, and added different concentrations of Doxycycline (hereinafter referred to as Doxycycline) to the cell culture medium. "Dox" for short) induced the expression of RIPK3-2×FKBP protein, and then performed Western blot experiments, figure 1 It is a schematic diagram showing that the expression of RIPK3-2×FKBP depends on Dox; the results show that in A549 and Hela cells, the expression of RIPK3-2×FKBP is very high when the concentration of Dox is 1 μg / mL, while in the control without adding DOX The expression of RIPK3-2×FKBP was not detected in the cells. Meanwhile, the induction effect of 1μg / mL DOX in Hela was even better than that of 4μg / mL and 10μg / mL Dox. These r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com